3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(6):467-471. doi:10.7150/ijms.4639 This issue Cite
Research Paper
Zn2+-SCMC Versus HA for Preventing Intraperitoneal Adhesions: A Rat Model Study
1. Departments of Hepatobiliary Surgery, the Norman Bethune First Hospital of Jilin University, Jilin 130021, China;
2. Departments of Anesthesiology, the Norman Bethune First Hospital of Jilin University, Jilin 130021, China;
3. Departments of Medical Statistics, the Norman Bethune First Hospital of Jilin University, Jilin 130021, China;
4. Departments of Emergency Surgery, the Norman Bethune First Hospital of Jilin University, Jilin 130021, China.
Received 2012-5-23; Accepted 2012-7-24; Published 2012-8-9
Abstract
Background: Intraperitoneal adhesion is a serious surgical postoperative complication. Using a rat model, we compared the effectiveness of intraperitoneally administered zinc-modified sodium carboxymethyl cellulose (Zn2+-SCMC) and hyaluronic acid (HA) in preventing postoperative intraperitoneal adhesions.
Materials and Methods: Peritoneal adhesions were induced in 120 Wistar rats by scraping the cecal mucosa. The rats were randomized into a no treatment group (n = 40) or into a treatment group in which 3 ml of HA (n = 40) or Zn2+-SCMC (n = 40) was administered intraperitoneally before the abdominal wall was closed. Following sacrifice two weeks later, the intraperitoneal adhesions were scored and tissues were examined histologically using HE staining.
Results: Eight animals died, five in the untreated group (mortality rate, 12.5%), two in the HA group (mortality rate, 5.0%) and one in the Zn2+-SCMC group (mortality rate, 2.5%). Relative to the untreated group, the incidence of intraperitoneal adhesions was 77.5% in the HA and 48.7% in the Zn2+-SCMC group, with the incidence significantly lower in the Zn2+-SCMC group (P < 0.001). Both agents prevented intraperitoneal adhesions by promoting the repair of the abdominal serosa.
Conclusions: Administration of Zn2+-SCMC was more effective in preventing intraperitoneal adhesions than HA.
Keywords: Zn2+-SCMC, HA, intraperitoneal adhesion, abdominal surgery.
Introduction
Abdominal surgery is often associated with postoperative peritoneal adhesions, which may lead to significant clinical and economic consequences [1]. Among the serious postoperative complications associated with the formation of adhesions are chronic pelvic pain [2], primary and secondary infertility in women [3] and intestinal obstructions [4].
Methods to prevent postoperative adhesions have included modifications in surgical approaches and techniques and the use of surgical adjuvants [5]. Among the adjuvants tested in the prevention of postoperative adhesions are fibrinolytic agents, such as thrombokinase and fibrinolysin; anticoagulants such as heparin; anti-inflammatory agents such as aspirin and retinoic acid; and mechanical separation agents, such as carboxymethyl cellulose (CMC) and hyaluronic acid (HA) [6]. Peritoneal instillates that separate raw peritoneal surfaces during the first few days of the healing process may be an ideal approach for preventing postoperative adhesions.
Hyaluronic acid (HA), a naturally occurring polysaccharide composed of repeat disaccharide units of D-glucuronic acid and N-acetylglucosamine [7], has shown benefits in preventing adhesions following abdominal surgery [8, 9]. But a major limitation of HA is its high cost [10,11]. Intraperitoneal infusion of sodium CMC (SCMC) has also been utilized to prevent postoperative adhesions in various species, including rodents [12, 13], rabbits [14] and ponies [15]. Moreover, a bioresorbable membrane composed of HA and CMC has been approved by the United States Food and Drug Administration for the prevention of peritoneal adhesions, although there are other antiadhesive films, such as Statofilm, that have better results in rats, than Seprafilm® (Genzyme Biosurgery, Cambridge MA, USA) 10,11]. Limitations in the handling of HA/CMC have led to the addition of glycerol (G-HA/CMC), improving its tensile strength [16-17]. A prospective, randomized, evaluator-blinded multicenter trial showed that G-HA/CMC could effectively reduce adhesions to midline incisions and adhesions between the omentum and small bowel after abdominal surgery [18]. Another limitation in the use of HA and SCMC are their short resorption times in the abdomen. For example, the half-life of HA has been reported to range from 3 to 5 days [19], and, in rats, SCMC could be resorbed within 5 days (unpublished data). Zinc modified SCMC (Zn2+-SCMC) was developed to prolong the resorption period. Using a rat model, we have compared the effectiveness of Zn2+-SCMC and HA in preventing intraperitoneal adhesions.
Materials & Methods
Reagents
Zn2+-SCMC and HA were purchased from the Changchun Institute of Applied Chemistry of the Chinese Academy of Sciences, Changchun, China.
Animals
A total of 120 male and female Wistar rats, weighing 220±40 g, were obtained from the Experimental Animal Center of Norman Bethune College of Medicine, Jilin University, Changchun, China. The animal use and care protocols were reviewed and approved by the Animal Use Committee of Jilin University and were in accordance with our institutional guidelines for animal care and the Guide for Care and Use of Laboratory Animals published by the United States National Institutes of Health.
Surgery and experimental assignment
Rats were anesthetized with ethyl ether and fixed on the operating table in a supine position. A 4 cm vertical incision was made in the midline of the lower abdomen. The cecum was exteriorized using atraumatic forceps, and a scalpel was used to scrape a 0.3 × 12 cm area of the ileal serosa about 15 cm from the ileocecal valve. Animals were randomized into a no treatment group (n = 40), a HA treatment group (n = 40), or a Zn2+-SCMC treatment group (n = 40). Rats in the untreated group received no further treatment, whereas rats in the HA and Zn2+-SCMC groups received injections of 3 ml of 3% HA or 3 ml of 3% Zn2+-SCMC onto the area of incision area. The molecular weight of CMC is 17000 and the concentrations of both HA and Zn2+-SCMC were optimized by our prelimitary experiments. The incisions were then closed in several layers. The rats were deprived of food for 10 h, housed separately and allowed to recover.
Evaluation of the intraperitoneal adhesions
Intraabdominal adhesions were determined immediately after death or after sacrifice two weeks postoperatively using the Nair Score [20]: 0 indicated a complete absence of adhesions; 1 indicated a single band of adhesions, between viscera or from the viscera to the abdominal wall; 2 indicated two such bands of adhesions; 3 indicated more than two bands between the viscera, from the viscera to the abdominal wall or throughout the intestine, forming a mass that did not adhere to the abdominal walls; and 4 indicated viscera directly adhering to the abdominal wall, irrespective of the number and extent of adhesive bands. The percentage of rats in each group with adhesions (scores of 1-4) was calculated relative to the total number of rats in that group.
Histological examination
Areas of adhesion were carefully removed, fixed in 4% paraformaldehyde (PFA), dehydrated with a graded ethanol series, cleared in dimethylbenzene and embedded in paraffin. Sections of thickness 5 μm were deparaffinized by immersing in dimethylbenzene and rehydrated. The sections were stained with hematoxylin and eosin (HE) using standard procedures and analyzed under a light microscope.
Statistical analysis
Data were analyzed by SAS 9.0 software. The adhesion score was compared by rank-sum test among three groups. Fisher's exact test was used to compare the percentage of rats with adhesions among three groups. P < 0.05 was recognized as statistical different.
Results
Effects of Zn2+-SCMC and HA on animal survival
Five animals in the untreated group died (mortality rate 12.5%), compared with only two rats in the HA (mortality rate, 5.0%) group and one in the Zn2+-SCMC (mortality rate, 2.5%) group. Autopsies showed that the animals in the untreated and HA groups died of tissue necrosis, induced by severe bowel adhesion and subsequent local strangulation. However, no obvious bowel adhesions in the abdominal cavity were observed in the Zn2+-SCMC treated rat that died. Rather, the cause of death was traumatic shock induced by the perforation of the stripped ileum. The remaining rats survived 14 days after surgery, and all showed complete wound healing.
Effects of Zn2+-SCMC and HA on intraperitoneal adhesions
In the untreated group, eight animals developed degree 1 and degree 2 adhesions, and 12 developed degree 3 and degree 4 adhesions (Table 1). Of the 40 rats in the HA group, nine showed no adhesions (degree 0), and eight, 15, six and two animals developed degree 1, 2, 3 and 4 adhesions, respectively. HA significantly reducing adhesions compared with the untreated group (P < 0.001). The incidence of adhesions was further reduced in the Zn2+-SCMC group (P < 0.001 compared with the untreated and HA groups). Of the 40 animals in this group, 20 did not develop adhesions (degree 0), 12 developed degree 1 adhesions and seven developed degree 2 adhesions.
Formation of intraperitoneal adhesions in untreated, Zn2+-SCMC-treated and HA-treated rats.
| Group | N | Adhesion score | Percentage of rats with adhesions | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |||
| Untreated | 40 | 0 | 8 | 8 | 12 | 12 | 100 |
| HA | 40 | 9 | 8 | 15 | 6 | 2 | 77.5 |
| Zn2+-SCMC | 39 | 20 | 12 | 7 | 0 | 0 | 48.71 |
Adhesion score: HA vs. Untreated, P=0.0024; Zn2+-SCMC vs. Untreated, P < 0.001; Zn2+-SCMC vs. HA, χ2 =7.04, P=0.0080
H=46.84, P < 0.001, HA vs. Untreated, Z=3.83, p=0.0001; Zn2+-SCMC vs. Untreated Z=6.50 P < 0.001; Zn2+-SCMC vs. HA, Z=3.72, p=0.0002.
Zn2+-SCMC or HA prevented intraperitoneal adhesion by promoting serosal repair
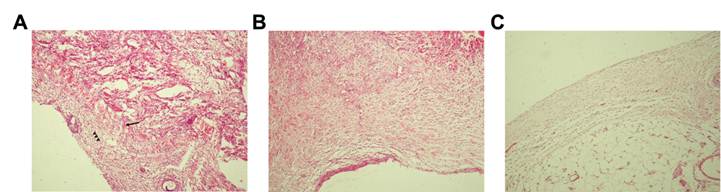
To investigate the mechanisms by which Zn2+-SCMC and HA prevented intraperitoneal adhesions, we examined these tissues histologically using HE staining. Adherent bowel segments in the untreated group showed increased fibroblast proliferation and a compact arrangement of collagen fibrils, with no repair of the injured abdominal serosa (Fig. 1A). However, serosal repair was observed in rats treated with HA (Fig. 1B) and Zn2+-SCMC (Fig. 1C). In both groups, the collagen fibrils were loosely arranged, and the interstitial cells were distributed in series. Fewer fibroblasts were observed in the Zn2+-SCMC animals compared to HA treated rats. These results indicated that Zn2+-SCMC and HA prevented intraperitoneal adhesion by promoting the repair of the abdominal serosa.
Photomicrographs of the adhesions. Representative sections of adhesion fibrous tissue were obtained from untreated (A), HA treated (B) and Zn2+-SCMC treated (C) rats and stained with HE. Collagen fibrils were indicated by arrows and fibroblasts were indicated by arrowheads. (A) Increased fibroblast proliferation and a compact arrangement of collagen fibrils appeared. No repair of the injured abdominal serosa was found. (B) The injured abdominal serosa was partially repaired. Interstitial cells were loosely arranged and the number of collagen fibrils and fibroblasts were relatively increased. (C) Collagen fibrils were loosely arranged, and the interstitial cells were distributed in series. Fewer fibroblasts were observed.
Discussion
The formation of postoperative peritoneal adhesions is an important obstacle in improving clinical outcomes following abdominal surgery [1]. At present, SCMC and HA are widely used to prevent postoperative intraperitoneal adhesion formation [8, 9, 16-17], and a bioreabsorbable physical barrier composed of HA/CMC (Seprafilm, Genzyme Biosurgery, Cambridge MA, USA) has been approved for clinical use [21, 22]. However, its relatively rapid resorption rate in the abdomen has hindered its ability to prevent intraperitoneal adhesions. We therefore investigated the ability of a cross-linked Zn2+-SCMC to prevent the formation of intraperitoneal adhesions. This novel barrier material retains most of the properties of SCMC, including its biodegradability, biocompatibility and high security. Moreover, this material is not cytotoxic, antigenic, or mutagenic and does not induce pyrogen reactions or obvious hemolysis. Due to its fluidity, this liquid gel can uniformly distribute within the peritoneal cavity. Our in vivo study showed that two days after surgery, 1.5-2.0 ml of the exudate was present in the abdominal cavity, whereas after an additional three days, the exudate was completely absorbed (data not shown). A thin film, 1.0 mm in thickness, was present between the injured parietal peritoneum and the injured intestine or between intestinal surfaces. This film remained visible seven days after surgery. Two weeks after the operation, some loose adhesive tissues were found between the injured parietal peritoneum and injured intestine, and the damaged serosa had recovered. These results suggest that Zn2+-SCMC was completely absorbed seven days after injury. As the half-life of HA is 3-5 days [19] and SCMC is absorbed within 5 days in rats (unpublished data), zinc crosslinking may prolong the absorption rate of SCMC, resulting in a suitable biodegradation rate in vivo.
We found that Zn2+-SCMC treatment significantly reduced intraperitoneal adhesions compared with HA treatment (48.71% vs. 77.5%, P < 0.001). Assessments of Nair scores showed that none of the rats administered Zn2+-SCMC had severe adhesions. On the other hand, untreated rats had adhesion scores of 3-4 and HA treated rats had scores of 2. These findings indicated that Zn2+-SCMC was more effective than HA in preventing intraperitoneal adhesions. In addition, all rats showed complete healing of incisions, suggesting that Zn2+-SCMC did not affect the wound closure process in these animals.
SCMC has been shown to suppress adhesion formation during the regeneration of epithelial tissues, to inhibit the migration of inflammatory cells during repair of damaged peritoneum, to decrease the viability of fibroblasts, and to reduce the accumulation of fibroblasts in injured peritoneum, thus limiting postoperative contact between tissues [23-25]. Histological examination of Zn2+-SCMC treated rats showed that collagen fibrils were loosely arranged and the interstitial cells distributed in series. Furthermore, the number of fibroblasts was much lower in Zn2+-SCMC- than in HA-treated rats, suggesting that Zn2+-SCMC prevented intraperitoneal adhesions by suppressing the accumulation of fibroblasts at injured surfaces.
In summary, our results indicated that Zn2+-modified SCMC was more effective than HA in preventing intraperitoneal adhesions in Wistar rats. Taken together, our results suggest that Zn2+-modified SCMC may be a suitable barrier for clinical use. Further studies, however, are needed to determine the safety and long-term effectiveness of this adhesion barrier in abdominal surgery. The effect of Zn2+-SCMC in preventing postoperative intraperitoneal adhesions under septic conditions also needs to be investigated in future study.
Acknowledgements
The authors thank Medjaden Bioscience Limited for assistance in the preparation of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Restrepo C, Chacon J, Manjarres G. Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int. 2010;30(6):619-25
2. Howard FM. The role of laparoscopy in the chronic pelvic pain patient. Clin Obstet Gynecol. 2003;46(4):749-66
3. D'Cruz OJ, Uckun FM. Targeting mast cells in endometriosis with janus kinase 3 inhibitor, JANEX-1. Am J Reprod Immunol. 2007Aug;58(2):75-97
4. Harris DA, Topley N. Peritoneal adhesions. Br J Surg. 2008;95(3):271-272
5. Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):111-8
6. Lim R, Stucchi AF, Morrill JM, Reed KL, Lynch R, Becker JM. The efficacy of a hyaluronate-carboxymethylcellulose bioresorbable membrane that reduces postoperative adhesions is increased by the intra-operative co-administration of a neurokinin 1 receptor antagonist in a rat model. Surgery. 2010Nov;148(5):991-9
7. Di Simone MP, Baldi F, Vasina V, Scorrano F, Bacci ML, Ferrieri A, Poggioli G. Barrier effect of Esoxx(®) on esophageal mucosal damage: experimental study on ex-vivo swine model. Clin Exp Gastroenterol. 2012;5:103-7
8. Micheels P, Besse S, Flynn TC, Sarazin D, Elbaz Y. Superficial dermal injection of hyaluronic Acid soft tissue fillers: comparative ultrasound study. Dermatol Surg. 2012Jul;38(7 Pt 2):1162-9
9. Rzany B, Cartier H, Kestemont P, Trevidic P, Sattler G, Kerrouche N, Dhuin JC, Ma YM. Full-Face Rejuvenation Using a Range of Hyaluronic Acid Fillers: Efficacy, Safety, and Patient Satisfaction over 6 Months. Dermatol Surg. 2012Jul;38(7 Pt 2):1153-61
10. Lalountas M, Ballas K, Skouras C, Asteriou C, Kontoulis T, Pissas D, Triantafyllou A, Sakantamis A. Preventing intraperitoneal adhesions with atorvastatin and sodium hyaluronate/carboxymethylcellulose: a comparative study in rats. The American Journal of Surgery. 2010;200:118-23
11. Lalountas M, Ballas K, Michalakis A, Psarras K, Asteriou C, Giakoustidis D, Nikolaidou C, Venizelos I, Pavlidis T, Sakantamis A. Postoperative adhesion prevention using a statin-containing cellulose film in an experimental model. British Journal of Surgery. 2012;99(3):423-9
12. Kutlay J, Ozer Y, Isik B, Kargici H. Comparative effectiveness of several agents for preventing postoperative adhesions. World J Surg. 2004Jul;28(7):662-5
13. Wang D, Mo J, Pan S, Chen H, Zhen H. Prevention of postoperative peritoneal adhesions by O-carboxymethyl chitosan in a rat cecal abrasion model. Clin Invest Med. 2010Aug1;33(4):E254-60
14. Cui H, Gensini M, Kataria R, Twaddle T, Zhang J, Wadsworth S, Petrilli J, Rodgers K, diZerega G, Cooper K. Reducing post-surgical adhesions utilizing a drug-enhanced device: sodium carboxymethylcellulose aqueous gel/poly(p-dioxanone) and Tranilast. Biomed Mater. 2009Feb;4(1):015001
15. Murphy DJ, Peck LS, Detrisac CJ, Widenhouse CW, Goldberg EP. Use of a high-molecular-weight carboxymethylcellulose in a tissue protective solution for prevention of postoperative abdominal adhesions in ponies. Am J Vet Res. 2002Oct;63(10):1448-54
16. Mukai T, Kamitani S, Shimizu T, Fujino M, Tsutamoto Y, Endo Y, Hanasawa K, Tani T. Development of a novel, nearly insoluble antiadhesive membrane. Eur Surg Res. 2011;47(4):248-53
17. Beck DE, Cohen Z, Fleshman JW, Kaufman HS, van Goor H, Wolff BG. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46(10):1310-9
18. Cohen Z, Senagore AJ, Dayton MT, Koruda MJ, Beck DE, Wolff BG, Fleshner PR, Thirlby RC, Ludwig KA, Larach SW, Weiss EG, Bauer JJ, Holmdahl L. Prevention of postoperative abdominal adhesions by a novel, glycerol/sodium hyaluronate/carboxymethylcellulose-based bioresorbable membrane: a prospective, randomized, evaluator-blinded multicenter study. Dis Colon Rectum. 2005;48(6):1130-9
19. Cencetti C, Bellini D, Longinotti C, Martinelli A, Matricardi P. Preparation and characterization of a new gellan gum and sulphated hyaluronic acid hydrogel designed for epidural scar prevention. J Mater Sci Mater Med. 2011Feb;22(2):263-71
20. Nair SK, Bhat IK, Aurora AL. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch Surg. 1974;108(6):849-853
21. Stepanian S. The results of use of the antiadhesive seprafilm barrier in adhesive disease of abdomen]. Georgian Med News. 2011May(194):12-8
22. Fazio VW, Cohen Z, Fleshman JW, van Goor H, Bauer JJ, Wolff BG, Corman M, Beart RW Jr, Wexner SD, Becker JM, Monson JR, Kaufman HS, Beck DE, Bailey HR, Ludwig KA, Stamos MJ, Darzi A, Bleday R, Dorazio R, Madoff RD, Smith LE, Gearhart S, Lillemoe K, Gohl J. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49(1):1-11
23. Fogle CA, Gerard MP, Elce YA, Little D, Morton AJ, Correa MT, Blikslager AT. Analysis of sodium carboxymethylcellulose administration and related factors associated with postoperative colic and survival in horses with small intestinal disease. Vet Surg. 2008Aug;37(6):558-63
24. Eggleston RB, Mueller PO, Parviainen AK, Groover ES. Effect of carboxymethylcellulose and hyaluronate solutions on jejunal healing in horses. Am J Vet Res. 2004;65(5):637-43
25. Cui H, Gensini M, Kataria R, Twaddle T, Zhang J, Wadsworth S, Petrilli J, Rodgers K, diZerega G, Cooper K. Reducing post-surgical adhesions utilizing a drug-enhanced device: sodium carboxymethylcellulose aqueous gel/poly(p-dioxanone) and Tranilast. Biomed Mater. 2009Feb;4(1):015001
Author contact
![]() Corresponding author: Dr. Guohui Liu, Emergency Surgery, the Norman Bethune First Hospital of Jilin University, Jilin 130021, China. Email: jlyuedu.cn. Tel: 86-0431-88782478; Fax: 86-0431-88782222.
Corresponding author: Dr. Guohui Liu, Emergency Surgery, the Norman Bethune First Hospital of Jilin University, Jilin 130021, China. Email: jlyuedu.cn. Tel: 86-0431-88782478; Fax: 86-0431-88782222.

 Global reach, higher impact
Global reach, higher impact