Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(5):327-333. doi:10.7150/ijms.4222 This issue Cite
Research Paper
A Comparison of Ketamine and Paracetamol for Preventing Remifentanil Induced Hyperalgesia in Patients Undergoing Total Abdominal Hysterectomy
1. Kanuni Sultan Suleyman Training and Research Hospital, Department of Anesthesia and Intensive Care, Istanbul, Turkey;
2. Konya University, Meram Medical Faculty, Department of Anesthesia and Intensive Care, Konya, Turkey.
Received 2012-2-10; Accepted 2012-6-5; Published 2012-6-20
Abstract
Background: The aim of this prospective, randomized, placebo-controlled study was to compare the effects of ketamine and paracetamol on preventing remifentanil induced hyperalgesia.
Methods: Ninety patients undergoing total abdominal hysterectomy were randomly assigned to one of three groups to receive (I) either saline infusion; (II) 0.5 mg/kg ketamine iv bolus or (III) 1000 mg iv paracetamol infusion before induction of anesthesia. Until the skin closure, anesthesia was maintained with 0.4 µg/kg/min remifentanil infusion in all groups, additionally Group II received 5 µg/kg/min ketamine infusion. Pressure pain thresholds were measured the day before surgery during the preoperative visit for baseline measurements and repeated postoperatively at 24 and 48 hours (hrs). Pressure pain thresholds were established by digital algometer on three different peri- incisional regions for calculating mean pressure pain threshold values. The visual analogue scale (VAS), sedation scores, total morphine consumption and side effects were assessed postoperatively.
Results: Demographic characteristics, duration of surgery and anesthesia were similar in the three groups. Pain thresholds at the incision region were significantly lower at 24 and 48 hrs postoperatively in Group I than the other Groups (p< 0.05). In Group І, pain thresholds were lower compared with preoperative baseline values. Thresholds in Group ІІ and Group ІІІ were higher compared with preoperative baseline values (p< 0.05) The VAS scores at all evaluation times were significantly higher in Group І when compared to Group ІІ and at 2, 4, 6 ,12 hrs were higher in Group I than Group ІІІ (p< 0.05). The morphine consumption was higher in Group ІІІ at 24 and 48 hrs postoperatively (p< 0.05).
Conclusion: It was shown that ketamine and paracetamol were both effective in preventing remifentanil induced hyperalgesia.
Keywords: remifentanil, ketamine, paracetamol, postoperative pain, hyperalgesia.
Introduction
Opioids are potent analgesics that are often necessary for treating moderate to severe pain. However, experimental studies report that opioids may also elicit hyperalgesia and allodynia (1). It is, therefore, likely that tolerance develops more rapidly with a rapid offset drug such as remifentanil than with longer acting opioids (2). The most likely explanation for the greater postoperative analgesic requirement for remifentanil is development of acute opioid tolerance to morphine analgesia (3). Opioid-induced processes that underlie hyperalgesia reduce antinociception and contribute to opioid tolerance (4-6).
Among the potential mechanisms leading to opioid induced hyperalgesia and antinociceptive tolerance, N-methyl-D-aspartate (NMDA) pain-facilitator processes seem to play a key role (1,7,8). Experimental studies performed in animals and volunteers have shown that NMDA receptor antagonists such as ketamine inhibit central sensitization and prevent opioid induced hyperalgesia (9-12).
The analgesic and antihyperalgesic actions of cyclooxygenase (COX) inhibitors, the so-called non-steroidal anti-inflammatory drugs (NSAIDs), have traditionally been attributed to inhibition of peripheral prostaglandin (PG) synthesis in inflamed tissue (13). However, there is increasing evidence that at least part of their analgesic effects depends on COX inhibition in the central nervous system (14). Both isoforms are constitutively expressed in the rat brain and spinal cord (15). Recently, a third distinct isoform, COX-3, has been described, which is a spliced COX-1 variant and is suggested to represent the primary central mechanism by which paracetamol (acetaminophen) decreases pain and possibly fever (13). In the last decade, several peripheral antihyperalgesic actions of NSAIDs have been demonstrated in human models of mechanical and heat hyperalgesia (16). In rats, there is also evidence for COX-induced central sensitization. Also, in humans, the rapid onset of analgesic effects of COX-2 inhibitors after brief surgical intervention suggest a central antihyperalgesic effect, but direct evidence for this action is still lacking (15,17).
In humans, opioid tolerance, the analgesic effects of opioids and opioid requirements are evaluated with a quantitative sensorial test (QST) (18-20). It was shown that ketamine prevented postoperative hyperalgesia induced by remifentanil (1). However, we did not find the effects of paracetamol on remifentanil induced hyperalgesia in the postoperative setting. Therefore, we planned to test the effects of paracetamol on remifentanil-induced hyperalgesia and compare these with ketamine, which has been shown to prevent remifentanil-induced hyperalgesia, by using postoperative pain scores, opioids consumption and quantitative sensorial test.
Materials & Methods
After receiving approval from Ethical Committee of Selcuk University Meram Medical Faculty, Konya, Turkey (Ethical Committee B.30.2.SEL. 002.0081-2917, 30 April 2008) and written informed consent, we enrolled 90 patients of ASA physical status I-II scheduled for elective total abdominal hysterectomy by using a computer-generated random number system. Patients with a history of psychiatric disorders, chronic pain, renal, cardiac or hematological insufficiency, chronic analgesic or opioid treatment, aged below 35 yr and above 70 yr, inability to use a patient-controlled analgesia (PCA) device and duration of surgery over 120 min were excluded from the study. During the preoperative visit, the day before surgery, all patients were instructed in the use of the 10-step visual analogue scale (VAS; 0 = no pain, 10 = greatest imaginable pain), PCA device (Abbott Pain Management Provider, Chicago), and quantitative sensory tests (QST) applied with a digital pressure algometer (Chatillon DFE-100, Digital Force Gauge/AMETEK) by an anesthesiologist. Additionally, baseline values for QST on skin area of surgery were performed. A handheld digital pressure algometer with a 1 cm2 probe area was used to determine pressure pain threshold. The patients informed the researcher when pain was perceived and the researcher immediately pushed a button to freeze the digital display. The first pressure value at which pain was registered was saved as Lb unit value. The average of three measurements with an interstimulus interval of 60 s was defined as the pressure pain threshold value. Pressure pain thresholds were measured in an area 2-3 cm from the incision at three levels (top, middle, and bottom; baseline values) and on the inner forearm (control values). A mean value for the three peri-incisional regions was calculated and used for statistical comparisons. The, QSTs were repeated at 24 and 48 hours postoperatively.
All patients were premedicated with 10 mg oral diazepam the night before surgery and 10 mg intramuscular diazepam one hour before surgery. Patients were randomly assigned to one of the three groups using a computer-generated random numbers. Baseline heart rate, systolic (SAP), diastolic (DAP) and mean arterial pressure (MAP) were recorded before induction of anesthesia and at 15 min intervals during surgery. Patients in Group I received physiologic saline; whereas those in Group II received intravenous (iv) bolus ketamine 0.5 mg/kg, and those in Group III received 1000 mg paracetamol (infusion/15 min) before the induction of anesthesia. The patients in Group II also received a maintenance infusion of 5 µg/kg/min ketamine intraoperatively until skin closure.
General anesthesia was induced with remifentanil 1 µg/kg and propofol 1.5-2 mg /kg followed by atracurium 0.5 mg/kg to facilitate tracheal intubation. Anesthesia was maintained with 0.4 µg/kg/min remifentanil infusion and desflurane 0.5 MAC. Lungs were mechanically ventilated (end-tidal CO2 values of 35-40 mmHg) with 50% air in an oxygen mixture. All patients in the three groups had received the same anesthesia regimen. Insufficient anesthesia was defined as a heart rate that exceeded pre-induction values by 15% and SAP exceeding baseline values by 20% for at least 1 min. Patient movement, coughing, tearing and sweating were also considered signs of inadequate anesthesia. Inspired desflurane was increased stepwise by 1% MAC when insufficient anesthesia was suspected. Hypotension, defined by a MAP less than 60 mmHg, prompted stepwise 1% MAC reductions in desflurane. If bradycardia and hypotension persisted, additional iv fluids, atropine and ephedrine were also given. Thirty minutes before the anticipated end of surgery, a 0.15 mg/kg bolus dose of morphine was given intravenously.
After skin closure, desflurane, remifentanil and ketamine infusion were discontinued, and residual neuromuscular blockade was reversed by 0.04-0.08 mg/kg iv neostigmine and 0.02-0.04 mg/kg iv atropine. The trachea was extubated when patients responded to the verbal commands, spontaneous respiratory rate exceeded 12 breaths/min, and end-tidal carbon dioxide partial pressure was less than 45 mmHg. The times from the remifentanil discontinuation until awakening (awakening time) and tracheal extubation (extubation time) were recorded.
When patients responded to verbal commands, the first postoperative VAS was taken and noted as VAS 0 hr. Another observer, who was unaware of patients' group assignments, evaluated patients during the postoperative period. When VAS score was less than 5, patients were connected to a PCA device set to deliver 1 mg morphine as an iv bolus with a 6-min lockout interval; continuous infusion was not allowed. This PCA regimen was continued for 48 hrs after tracheal extubation and other analgesics were not used during this period. The VAS scores, analgesic demand, analgesic delivery, morphine consumption and sedation scores (1: patient fully awake, 2: patient occasionally asleep, 3: patient often sleep but awakening easily 4: difficulty awakening, 5: not awakening) were recorded at 2, 4, 6, 12 and 24 hrs postoperatively. Any adverse postoperative effects, such as nausea-vomiting, nightmare, diplopia, hallucination or agitation were noted. The satisfaction with analgesia of the patients was graded on a four-point scale (1-4) as follows: 1, poor; 2, intermediate; 3, good; 4, excellent.
Statistical Analysis
All analyses were conducted using SPSS software (Statistical Package for the Social Sciences, version 13.0, SPSS Inc, Chicago, IL, USA). Data was reported as mean ± standard deviation and the number n (%). One-way ANOVA (analysis of variance) was used for comparison between groups. Paired group-wise tests were performed to find groups that make a difference. Kruskal Wallis analysis was performed for the variables which were not included in the variance analysis. P values < 0.05 were considered significant.
Results
Ninety patients were enrolled in the study. Eleven patients were excluded due to postoperative fever, duration of surgery and non-cooperation. Twenty-seven patients were randomly assigned to Group І (control), twenty-six to Group ІІ (ketamine) and twenty-six to Group ІІІ (paracetamol). Demographic characteristics, duration of surgery and anesthesia were similar in the three treatment groups. Awakening time and extubation time were compared in the three groups, and they were significantly longer in Group II than other groups. (p< 0.05) (Table 1).
Intraoperatively desflurane requirement, SAP, DAP, MAP and heart rate were similar in the three groups. Three patients in Group І, one in Group ІІ and three in Group ІІІ required 0.5 mg atropine treatment (p > 0.05).
Patient characteristics and intraoperative variables. Values are shown as number of patients or mean ± SD.
| Groups | Group І (n = 27) | Group ІІ (n = 26) | Group ІІІ (n= 26) |
|---|---|---|---|
| Age (yr) | 48.14 ± 5.98 | 48.26 ± 5.66 | 47.2 ± 5.59 |
| Weight (kg) | 70.29 ± 11.5 | 73.34 ± 8.80 | 76.92 ± 7.89 |
| ASA-PS I/II/III (n) | 18/7/2 | 19/6/1 | 16/9/1 |
| Duration of anesthesia (min) | 80.55 ± 13.14 | 80.00 ± 13.41 | 80.38 ± 13.26 |
| Duration of surgery (min) | 70.55 ± 12.14 | 70.00 ± 13.41 | 70.38 ± 13.26 |
| Extubation time (sec) | 243.26 ± 64.01 | 317.42 ± 75.24* | 265.61 ± 60.46 |
| Awakening time (sec) | 260.44 ± 62.20 | 413.30 ± 26.88* | 282.23 ± 57.18 |
* p< 0.05 (Comparison between groups)
ASA-PS American Society of Anesthesiologists physical status
Postoperative VAS values of the Groups (mean±SD). * p< 0.05; Group I vs Group II; † p< 0.05; Group I vs Group III.
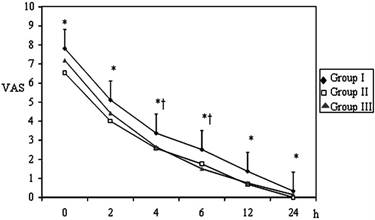
Pain VAS scores at 0, 2, 4, 6, 12 and 24 hrs postoperatively were assessed in all groups (Figure 1). The VAS scores at all evaluation times were significantly higher in Group І when compared to patients in Group ІІ and at 2, 4, 6 ,12 hrs were higher in Group I than Group ІІІ (p < 0.05). The VAS scores between Group ІІ and Group ІІІ were similar (p > 0.05).
At all of the postoperative evaluation times, analgesic delivery was higher in Group І compared to Groups ІІ and ІІІ. Patients' analgesic delivery was significantly higher at the 2, 12, 24 and 48 hrs in Group ІІІ than Group ІІ (p< 0.05) (Table 2). Analgesic demand was significantly lower in Group ІІ compared to Groups І and ІІІ (Table 3). Analgesic requirements were significantly higher in Group І at all times than Group ІІ and at 4, 6, 12, 24 and 48 hrs than Group ІІІ (p < 0.05). Cumulative 24 and 48 hrs morphine consumption was higher in Group ІІІ than Group ІІ (35.34±13.71mg at 24 hr and 42.52±15.08 mg at 48 hr in Group II; 48.53±12.40 at 24 hr and 57.11±16.71 mg at 48 hr in Group III) (p < 0.05).
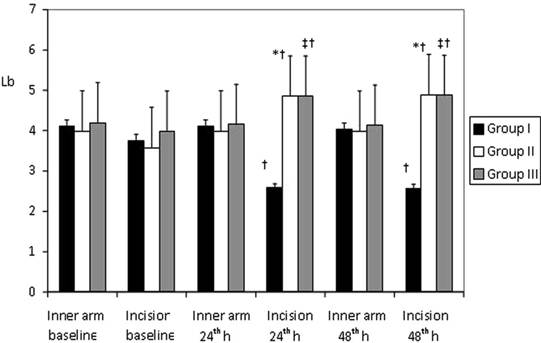
There were no significant differences between the Groups in terms of pain thresholds assessed with an algometer at 24 and 48 hrs and preoperatively on inner forearm. Pain thresholds at the incision region were significantly lower at 24 and 48 hrs postoperatively in Group I than in the other two Groups (p < 0.05). In Group І pain thresholds were lower compared with pre-operative baseline values; in Group ІІ and Group ІІІ pain thresholds were higher compared with pre-operative baseline values (p < 0.05) (Figure 2).
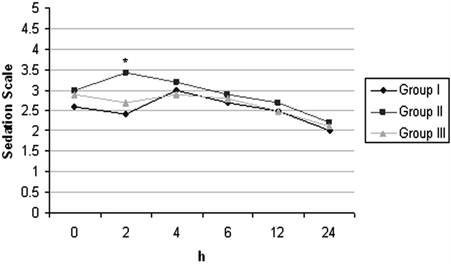
With respect to satisfaction scores, the patients in Group II and Group III were more satisfied than those in Group I and it was found to be statistically significant (p< 0.05) (Table 4). The incidences of nausea, vomiting and need for antiemetic treatment were similar in all groups. With respect to postoperative ketamine psychopharmacologic effects, two patients reported nightmare at 24 and 48 hrs after surgery and seven patients reported diplopia at 24 hrs. Sedation scores were similar in all groups but was higher in Group II only at postoperative 2nd hr. (Figure 3).
Analgesic delivery (mg of morphine consumption of the patients; the amount of infused and bolus doses of morphine with PCA device) of the patients. Values are shown as mean ± SD.
| Evaluation Times (hr) | Group І (n = 27) | Group ІІ (n = 26) | Group ІІІ (n= 26) |
|---|---|---|---|
| 2 | 15.66±2.63* | 12.15±3.0 | 14.03±3.56 |
| 4 | 26.11±4.57*† | 18.46±6.54 | 21.8±6.13 |
| 6 | 36.7±7.16*† | 23.53±8.96 | 28.15±8.36 |
| 12 | 57.07±15.49*† | 30.92±12.19 | 39.34±11.50 |
| 24 | 73.03±22.41*† | 35.34±13.71‡ | 48.53±12.40 |
| 48 (Total morphine dose) | 86.05±29.46*† | 42.52±15.08‡ | 57.11±16.71 |
* p< 0.05 (Group I vs Group II)
† p< 0.05 (Group I vs Group III)
‡ p< 0.05 (Group II vs Group III)
Analgesic demand (presses to the button of the PCA for delivery of morphine ) of the patients. Values are shown as mean ± SD.
| Evaluation Times (hr) | Group І (n = 27) | Group ІІ (n = 26) | Group ІІІ (n= 26) |
|---|---|---|---|
| 2 | 32.85±8.19*† | 23.0±7.08 | 28.69±7.01 |
| 4 | 53.03±12.19*† | 32.84±11.83 | 39.34±9.02 |
| 6 | 70.81±15.03*† | 40.61±15.56 | 49.11±11.70 |
| 12 | 102.44±27.49*† | 52.19±20.40 | 66.73±16.14 |
| 24 | 134.59±41.07*† | 60.11±23.74‡ | 92.69±20.44 |
| 48 | 146.19±21.2*† | 73.20±15.63‡ | 104.81±14.57 |
* p< 0.05 (Group I vs Group II)
† p< 0.05 (Group I vs Group III)
‡ p< 0.05 (Group II vs Group III)
Satisfaction scores of the patients. Values are shown as number of patients and percentage median.
| Satisfaction Score | Group І (n %) | Group ІІ (n %) | Group ІІІ (n %) |
|---|---|---|---|
| 1 | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 | 1 (3.7%) | 0 (0%) | 0 (0%) |
| 3 | 14 (51.85%) | 2 (7.7%) | 4 (15.4%) |
| 4 | 12 (44.4%) | 24 (92.3%)* | 22 (84.6%)* |
* p< 0.05 (Comparison between groups)
Mean Pressure Pain Thresholds (Lb) determined with digital pressure algometer on inner forearm and the surgical incision area at preoperative period and than postoperative 24th and 48th h (mean±SD ). * p< 0.05; Group I vs Group II; † p< 0.05; Group I vs Group III; ‡ p< 0.05; Group II vs Group III.
Postoperative Sedation Scores of the Groups (mean±SD). * p< 0.05 (Comparison between groups)
Discussion
The half-life of remifentanil is too short and therefore remifentanil is recommended for use as an infusion and is widely used as an important part of general anesthesia (3). Since the effect of duration is short, it is recommended to use additional analgesics before surgery ends to prevent postoperative pain. However, in these cases, earlier and more frequent use of first dose postoperative analgesic is connected to acute opioid tolerance (2, 11,21). In a study by Joly et al. using low (0.05 mg/kg/min) and high (0.4 mg/kg/min) doses of remifentanil, morphine consumption was higher in the high dose remifentanil group (1). This situation has been linked to development of rapid acute opioid tolerance and it is emphasized that the dose of remifentanil used was important (1). In line with this data, the current study used remifentanil at the dose of 0.4 mg/kg/min in order to better evaluate of the effectiveness of paracetamol and ketamine.
In hyperalgesia due to opioids, there is a relationship between reduction of antinociception and opioid tolerance (4-6,9). Under the guidance of potential mechanisms between antinociceptive tolerance and opioids induced hyperalgesia, NMDA is seen to play a key role in processes facilitating on pain (8). In experimental studies with volunteers, NMDA receptor antagonists such as ketamine inhibit central sensitization and have been shown to prevent opioid connected hyperalgesia (9-12). It was shown that low dose ketamine (0.5 mg/kg bolus and 0.5 mg/kg/min) when added to remifentanil, prevented remifentanil induced hyperalgesia (1). In line with this data, we elected to add low-dose ketamine (0.5 mg/kg bolus and 0.5 mg/kg/min infusion) to remifentanil infusion in our study.
Tolerance of opioids and hyperalgesia were evaluated with analgesic effect, opioid needs and quantitative sensory tests in several studies (9,18-20). QST, clinical and sensory evaluation are important experimental tools. In our study, postoperative pain scores and morphine consumption was characterized by an increase in hyperalgesia. The pressure pain threshold were assessed with digital pressure algometer.
In the current study, VAS scores were found to be significantly better in the ketamine group than control group and in accordance to this, morphine consumption was also found to be less than the control group. Decreased hyperalgesia was detected in the sensory test with a digital algometer, conducted at 24 and 48 hrs postoperatively in Group ІІ. Joly et al. used the same dose of remifentanil and ketamine as used in the present study, but pain scores and hyperalgesia in their study were not affected (1). We could explain this difference with the first bolus dose of ketamine use before the induction and use prior to the opioid implementation. Jaksch et al (22) used the same bolus dose of ketamine before the induction as we have implemented, but in their study postoperative pain were not affected. This is another different result from our study, and we believe his may be related to the high infusion dose usage in our study.
The peripheral anti-inflammatory and antihyperalgesic effects of NSAIDs have been shown in experimental and clinical studies (13, 23). Despite a large number of experimental studies related to central antihyperalgesic effects, there is a very limited number of clinical studies (13). In a study of central hyperalgesia models in rats, Bianchi and Paneri (24) evaluated the antihyperalgesic effects of lornoxicam, piroxicam and meloxicam, which have the same chemical structure but different COX-1 and COX-2 selectivities. All showed the same anti-inflammatory effect, did not cause changes in thermal nociceptive threshold, and significantly reduced hyperalgesia. However, only lornoxicam has been reported to be fully effective in prevention of hyperalgesia. The difference between anti-inflammatory and antihyperalgesic activities of NSAIDs and with the blocking of both COX-1 and COX-2 antihyperalgesic activity to be significantly reported (24). Peripheral inflammation, increased levels of spinal PG's, spinal PGE2, largely involved in the spinal nociceptive process and the increase in PGE2 concentration was shown to be correlated with hyperalgesia (24).
Different results have been reported on the antihyperalgesic effects of COX-3 inhibitors assumed as COX-1 variants and centrally effective paracetamol. In a study of volunteers, the antihyperalgesic effect of paracetamol (1000 mg) was evaluated and was shown to reduce secondary hyperalgesia field (13). In another study on volunteers, no antihyperalgesic effect of paracetamol (1000 mg) was reported (25). We did not come across a study evaluating the efficacy of paracetamol in preventing hyperalgesia due to intraoperative use of remifentanil. Therefore, in this study, paracetamol activity was compared with ketamine, supported by clinical studies, to prevent remifentanil induced hyperalgesia. We showed that VAS scores and morphine consumption was less in both the ketamine and paracetamol group. Additionally, reduced hyperalgesia was detected in the sensory test performed by digital algometer at 24 and 48 hrs postoperatively. These results show that paracetamol is also effective in preventing remifentanil induced hyperalgesia in humans, which has been known to contribute to secondary hyperalgesia.
The most important factor limiting the use of intraoperative and postoperative agents is undesirable effects. Ketamine may extend the period of awakening and extubation, may cause bad dreams, double vision, hallucinations, and agitation. It is also associated with dose-dependent incidence of side effects. In small doses (< 10 mg/hr), cognitive functions are not affected (26). In our study, in the ketamine group, during the early postoperative period, 7 patients had diplopia and 2 patients experienced bad dreams. The incidence of nausea and vomiting was similar between groups. Extubation and awakening time was longer in the ketamine group. Early postoperative sedation scores were higher in the ketamine group. The psychotomimetic reactions were not observed in the current study because of using low doses of ketamine. These low levels of ketamine do not usually cause side effects. We did not see any side effect associated with the use of paracetamol. Patient satisfaction was higher in the ketamine and paracetamol groups. We believe these results are based on better VAS scores.
In this study, we evaluated the effect of preemptive 1000 mg paracetamol on remifentanil-induced hyperalgesia in comparison with ketamine. Intraoperative hemodynamic parameters were not affected and no significant change in desflurane concentration was seen than ketamine group. In the postoperative period, pain scores and morphine consumption were lower in both the paracetamol and ketamine group.
In conclusion, concerning the effects of the drugs, paracetamol is as effective as ketamine in preventing hyperalgesia caused by the use of intraoperative remifentanil. Further studies comparing paracetamol with other drugs that have been shown to prevent opioid induced hyperalgesia are needed to confirm our results.
Competing Interests
None of the authors has any personal or financial relationship with the potential to inappropriately influence (bias) his or her actions or this manuscript; no financial or other potential conflicts of interest exist regarding this manuscript (includes involvement with any organization with a direct financial, intellectual, or other interest in the subject of the manuscript).
References
1. Joly V, Richebe R, Guignard B, Fletcher D, Maurette P, Sessler DI. et al. Remifentanil-induced Postoperative Hyperalgesia and Its Prevention with Small-dose Ketamine. Anesthesiology. 2005;103:147-155
2. Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P. et al. Acute Opioid Tolerance: Intraoperative Remifentanil Increases Postoperative Pain and Morphine Requirement. Anesthesiology. 2000;93:409- 417
3. Dershwitz M, Randel GI, Rosow CE, Fragen RJ, Connors PM, Librojo ES. et al. Initial clinical experience with remifentanil, a new opioid metabolized by esterases. Anesth Analg. 1995;81:619- 623
4. Vanderah TW, Gardell LR, Burgess SE, Ibrahim M, Dogrul A, Zhong CM. et al. Dynorphin promotes abnormal pain and spinal opioid antinociceptive tolerance. J Neurosci. 2000;20:7074-7079
5. Laulin JP, Celerier E, Larcher A, Le Moal M, Simonnet G. Opiate tolerance to daily heroin administration: an apparent phenomenon associated with enhanced pain sensitivity. Neuroscience. 1999;89:631-636
6. Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213-217
7. Feng J, Kendig JJ. N-methyl-D-aspartate receptors are implicated in hyperresponsiveness following naloxone reversal of alfentanil in isolated rat spinal cord. Neurosci Lett. 1995;189:128-30
8. Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance. A current view of their possible interactions. Pain. 1995;62:259-74
9. Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P. et al. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology. 2000;92:465-472
10. Kissin I, Bright CA, Bradley EL Jr. The effect of ketamine on opioid-induced acute tolerance. Can it explain reduction of opioid consumption with ketamine opioid analgesic combinations? Anesth Analg. 2000:483-488
11. Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49-57
12. Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schüttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152-159
13. Koppert W, Wehrfritz A, Körber N, Sittl R, Albrecht S, Schüttler J. et al. The cyclooxygenase isozyme inhibitors parecoxib and paracetamol reduce central hyperalgesia in humans. Pain. 2004;108:148- 153
14. Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136-146
15. Svensson CI, Yaksh T. The spinal phospholipase-cyclooxygenase- prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553-583
16. Wilgus TA, Ross MS, Parrett ML, Oberyszyn TM. Topical application of a selective cyclooxygenase inhibitor suppresses UVB mediated cutaneous inflammation. Prostaglandins Other Lipid Mediat. 2000;62:367-384
17. Seybold VS, Jia YP, Abrahams LG. Cyclooxygenase- 2 contributes to central sensitization in rats with peripheral inflammation. Pain. 2003;105:47-55
18. Curatolo M, Petersen -Felix S, Arendt NL, Zbinden AM. Epidural epinephrine and clonidine: segmental analgesia and effects on different pain modalities. Anesthesiology. 1997;87:785-794
19. Brennum J, Dahl JB, Moiniche S, Arendt- Nielsen L. Quantitative sensory examination of epidural anaesthesia and analgesia in man: effects of pre- and post-traumatic morphine on hyperalgesia. Pain. 1994;59:261-271
20. Pedersen JL, Kehlet H. Secondary hyperalgesia to heat stimuli after burn injury in man. Pain. 1998;76:377-384
21. Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998;86(6):1307-11
22. Jaksch W, Lang S, Reichhalter R, Raab G, Dann K, Fitzal S. Perioperative small-dose S(+)-ketamine has no incremental beneficial effects on postoperative pain when standard-practice opioid infusions are used. Anesth Analg. 2002;94(4):981-6
23. Bickel A, Dorfs S, Schmelz M, Forster C, Uhl W, Handwerker HO. Effects of antihyperalgesic drugs on experimentally induced hyperalgesia in man. Pain. 1998;76:317- 325
24. Bianchi M, Panerai AE. Effects of lornoxicam, piroxicam and meloxicam in a model of thermal hindpaw hyperalgesia induced by formalin injection in rat tail. Pharmacological Research. 2002;45:101-105
25. Chassaing C, Schmidt J, Eschalier A, Cardot JM, Dubray C. Hyperalgesia induced by cutaneous freeze injury for testing analgesics in healthy volunteers. Br J Pharmacol. 2006;61:389- 397
26. Himmelseher S, Durieux ME. Ketamine for perioperative pain management. Anesthesiology. 2005;102:211-220
Author contact
![]() Corresponding author: Hale BORAZAN, Konya University, Meram Medical Faculty, Department of Anesthesiology and Intensive Care, Akyokus, Meram, Konya/TURKEY. Fax: 090 332 2236181 Tel: 090 332 2237926 E-mail: borazanhcom
Corresponding author: Hale BORAZAN, Konya University, Meram Medical Faculty, Department of Anesthesiology and Intensive Care, Akyokus, Meram, Konya/TURKEY. Fax: 090 332 2236181 Tel: 090 332 2237926 E-mail: borazanhcom

 Global reach, higher impact
Global reach, higher impact