3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(4):311-315. doi:10.7150/ijms.4369 This issue Cite
Research Paper
Remifentanil Prevents Tourniquet-Induced Arterial Pressure Increase in Elderly Orthopedic Patients under Sevoflurane/N2O General Anesthesia
Department of Anesthesiology & Pain Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
Received 2012-3-15; Accepted 2012-6-4; Published 2012-6-8
Abstract
Aims: Prolonged tourniquet inflation produces a hyperdynamic cardiovascular response. We investigated the effect of continuous remifentanil infusion on systemic arterial pressure, heart rate, and cardiac output changes during prolonged tourniquet use in elderly patients under sevoflurane/N2O general anesthesia.
Methods: Thirty female patients scheduled for knee replacement arthroplasty were infused with either remifentanil at a target organ concentration of 2.0 ng/mL (remifentanil group, n = 15) or saline (control group, n = 15) after induction of anesthesia. Anesthesia was maintained with sevoflurane and N2O. Heart rate (HR), systolic arterial pressure (SAP), diastolic arterial pressure (DAP), cardiac index (CI), total systemic vascular resistance index (TSVRI), BIS, end-tidal sevoflurane concentration (EtSEVO), and end-tidal carbon dioxide concentration (EtCO2) were measured during the study period.
Results: There were significant differences in mean HR, SAP, DAP, and EtSEVO over time between the groups (P = 0.047, P < 0.001, P = 0.017, and P < 0.001, respectively). There was a statistically significant time trend effect (P < 0.001) in HR, SAP, DAP, and CI between the groups, with a statistically significant time-group interaction between the two groups (P = 0.02, 0.007, 0.001, 0.01, respectively).
Conclusion: The present study demonstrated that infusion with remifentanil prevented an increase in hemodynamic pressure during tourniquet inflation in elderly patients under sevoflurane/N2O general anesthesia.
Keywords: general anesthesia, knee replacement arthroplasty, remifentanil, tourniquet.
Introduction
Tourniquets are widely used during limb operations to minimize surgical bleeding and to maintain a relatively bloodless field. Exsanguination of the limb and inflation of the tourniquet produce an initial increase in arterial pressure. This increase has been attributed to several factors, including an expansion of central venous blood in association with a theoretical increase in peripheral vascular resistance and delayed hypertension, accompanied by ischemia and pain due to tourniquet compression [1-3]. These hemodynamic changes are minimal in healthy young patients but may not be tolerated by older patients with poor cardiac reserve [4]. Previous studies have only focused on the management of delayed tourniquet-induced hypertension in young patients [4-7].
Remifentanil is a selective µ-opioid receptor agonist with a rapid onset, short duration, and short blood/effect-site equilibration half-time. It is effective in preventing sympathetic responses induced by noxious stimuli. Some studies have indicated that remifentanil may be associated with significant hemodynamic changes characterized by decreases in arterial pressure, heart rate, cardiac output, and systemic vascular resistance [8,9]. Therefore, remifentanil have potential for prevention of both initial and delayed tourniquet-induced hypertension.
We investigated the effect of continuous intravenous remifentanil administration on systemic arterial pressure, heart rate, and cardiac output in elderly patients under sevoflurane/N2O general anesthesia who were undergoing knee surgery accompanied by use of a tourniquet.
Materials and Methods
This study was randomized, blinded, and prospective. The study protocol was approved by our Institutional Review Board and ethical committee. Written informed consent was obtained from each patient. Included in the study were 30 female patients ranging in age from 51 to 84 years with an ASA physical status of class I - II. Study participants were scheduled for total knee replacement surgery with a tourniquet under sevoflurane/N2O general anesthesia. Patients with ischemic heart disease and cerebrovascular disease were excluded. Fifteen patients each were assigned randomly to the control group and the remifentanil group using computer-generated sequence numbers.
Routine monitors were used including non-invasive arterial pressure, EKG, pulse oximetry, end-tidal CO2, and anesthetic concentrations. Induction of anesthesia was achieved with propofol 1.5 mg/kg administered intravenously. Muscle relaxation was induced with rocuronium 0.6 mg/kg administered intravenously. Anesthesia was maintained with sevoflurane. Laryngoscopy was performed following loss of all four twitches from the train-of-four test performed by ulnar nerve stimulation. All patients received mechanical ventilation with a mixture of nitrous oxide (50%) in oxygen (fresh gas flow rate = 3 L/min, inspiratory: expiratory ratio = 1:2). The inspired ventilation was adjusted to obtain an end-tidal CO2 partial pressure between 30 and 35 mmHg starting five min after anesthesia induction. This was maintained for the duration of the surgery. A 20-gauge catheter was placed in the radial artery for continuous blood pressure monitoring. In the remifentanil group, the infusion of remifentanil was started with a target organ concentration of 2.0 ng/mL via a target-controlled infusion system (Orchestra Module DPS, Fresenius-Vial, Brezins, France). In the control group, the infusion of saline was performed in the same fashion. The syringes used with both groups were labeled with remifentanil in order to blind the data collector. Heart rate (HR), systolic arterial pressure (SAP), diastolic arterial pressure (DAP), end-tidal carbon dioxide concentration (EtCO2), and end-tidal sevoflurane concentration (EtSEVO) were measured and recorded every ten minutes during the study period. Cardiac index (CI) and total systemic vascular resistance index (TSVRI) were monitored with transesophageal Doppler ultrasound monitor (EDUM; Hemosonic 100®; Arrow, Reading, PA, USA). A standard BIS monitor strip (BIS Sensor®, Aspect Medical Systems, Newton, MA, USA) was placed on the forehead before induction of anesthesia. The Bispectral index was displayed continuously throughout the procedure using a model A 2000 Spectral EEG monitor (Aspect Medical Systems, Natick, MA, USA) and maintained between 40 and 60. Vital signs taken before the inflation of the tourniquet were used as the baseline values. The concentration of sevoflurane was adjusted when necessary within the full range to maintain systolic arterial pressure and heart rate within 20% of baseline and to keep BIS values between 40 and 60. In all patients, the limb was exsanguinated with an elastic bandage before tourniquet inflation. A pneumatic tourniquet was applied to the thigh with a layer of “soft-roll” under the wrap. The width of the tourniquet cuff was 20 cm in all patients.
Escape medications were prepared for all patients. Ephedrine (3 mg increments) was available for hypotension (SAP < 80 mmHg for > 60 s), atropine (300 μg increments) was available for bradycardia (HR < 40 bpm), and esmolol (30 mg increments) was available for hypertension (SAP > 200 mmHg for > 60 s) or tachycardia (HR > 130 bpm for > 60 s). Patient data were excluded from further analysis if these drugs were given during the procedure.
A pilot study with eight patients showed a mean maximum SAP (SD) of 123 (8.7) and 147 (8.9) in the remifentanil group and the control group, respectively. Therefore, a sample size of five per group was calculated as being needed to show a difference of maximum SAP through 25 and 30 mmHg (SD 9 mmHg) between groups with an α risk of 0.01 and a β risk of 0.1 [7]. However, we recruited 15 patients for each group.
Student's t test or Fisher's exact test was used to analyze demographic data. HR, SAP, DAP, CI, TSVRI, EtCO2, EtSEVO, and BIS were analyzed by repeated measurement. All data are expressed as mean ± SD. P values of less than 0.05 were considered to be statistically significant.
Results
Age, weight, height, and preexisting medical conditions did not significantly differ between the groups (Table 1).
Demographic data.
| Group | ||
|---|---|---|
| Control | Remifentanil | |
| Patients (n) | 15 | 15 |
| Age (yr) | 69 ± 10 | 67 ± 8 |
| Weight (kg) | 65 ± 7 | 63 ± 9 |
| Height (cm) | 153 ± 4 | 150 ± 6 |
| Tourniquet time (min) | 87 ± 15 | 85 ± 14 |
| Hypertension (n) | 6 | 7 |
| Diabetes (n) | 2 | 1 |
Data are mean ± SD or n.
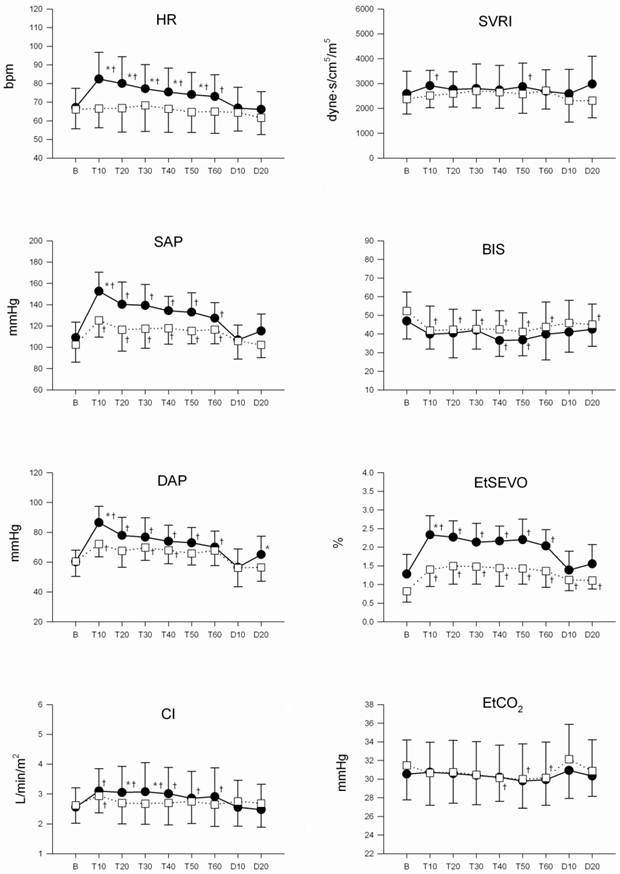
There were significant differences in mean HR, SAP, DAP, and EtSEVO during the procedure between the groups (P = 0.047, P < 0.001, P = 0.017, and P < 0.001, respectively). There were no differences in mean CI, TSVRI, EtCO2, or BIS over time between the groups.
HR, SAP, DAP, and CI demonstrated a statistically significant time trend effect (P < 0.001) with a statistically significant time-group interaction between the two groups (P = 0.02, 0.007, 0.001, 0.01) (Fig. 1). There were no circumstances that led to the withdrawal of a patient from the study.
Discussion
The present study demonstrates that an intravenous infusion of remifentanil significantly reduces hemodynamic increase during tourniquet inflation in elderly patients under sevoflurane/N2O general anesthesia. HR, SAP, DAP, and CI remained stable before, during, and after tourniquet inflation in the patients in the remifentanil group.
In healthy orthopedic patients, hemodynamic changes due to tourniquet inflation and deflation are minimal. However, tourniquet-induced hypertension can be a serious side effect in patients with cardiovascular problems, neurological diseases, or glaucoma [2,5]. We recruited elderly female patients for this study irrespective of the presence of hypertension or diabetes. Therefore, the outcome of this investigation could be clinically useful for the treatment of vulnerable patients compared with other studies using healthy subjects [5-7].
Hypotension after the induction of anesthesia is a well-recognized phenomenon during surgical preparations, such as draping, without any surgical stimuli and may be aggravated by deep anesthesia [10-12]. Anesthesiologists tend to lower the anesthetic depth during this period in order to avoid worsening the hypotension. The immediate increase in arterial pressure after inflation of a tourniquet may be due to light anesthesia, painful stimuli as a result of tourniquet inflation, an increase in systemic vascular resistance, or an expansion of central venous blood caused by exsanguination of the limb.
On the other hand, the delayed hypertension observed with the use of a tourniquet is known to be related to NMDA receptor activation by peripheral noxious stimuli from the extremities and occurs 40-60 min after inflation [5,6].
We monitored the CI and TSVRI of patients using esophageal Doppler ultrasonography throughout the study period. Esophageal Doppler ultrasonography offers continuous, noninvasive and reproducible cardiac output monitoring with less short-term variability than thermodilution [13,14]. In the control group of our study, CI increased by 19% ten minutes after tourniquet inflation and returned to near-baseline values after deflation. TSVRI increased by 13% ten minutes after inflation and was stable after deflation. Girardis et al. [3] demonstrated that CI did not change immediately after inflation but afterwards increased by 18% , with a further increase to a value 40% higher than baseline after deflation. They also demonstrated that systemic vascular resistance increased by 20% immediately after inflation with an 18% decrease after deflation. These differences from our findings can be explained by the fact that in the Girardis study, CI was calculated by the pulse contour method. In addition, their study investigated ten young men under propofol anesthesia. In contrast, Parmet et al. [15] reported a slight increase in CI with stable MAP and TSVRI values after tourniquet deflation in 34 patients undergoing knee arthroplasty during general anesthesia. The correspondence of these results to those obtained in our investigation can be explained by the similar nature of both studies. Both studies investigated elderly patients with cardiovascular disease undergoing total knee arthroplasty. Unlike our study, in the Parmet study, isoflurane concentration did not change from the time of tourniquet deflation until the conclusion of the study. These results can vary depending on the method of monitoring, the anesthetic drugs used, the type of operation, and the status of the patients enrolled in the study.
Changes in heart rate (HR), systolic arterial pressure (SAP), diastolic arterial pressure (DAP), cardiac index (CI), total systemic vascular resistance index (TSVRI), BIS, end-tidal sevoflurane concentraion (EtSEVO), end-tidal carbon dioxide concentration (EtCO2) during the surgery. Values are means (SD). Control group (●), and remifentanil group (□). B: baseline, T10-60: 10-60 min after tourniquet inflation, D10-20: 10-20 min after tourniquet deflation. * Changes from the baseline values were different (P < 0.05) between the groups. † Mean value was higher (P < 0.05) than at baseline within the same group.
Remifentanil stimulates NMDA receptors of different subunit combinations (NR1A/2A, NR1A/2B) via an allosteric mechanism leading to acute tolerance [16]. Sevoflurane, co-administered with remifentanil, inhibits NMDA receptors in a dose-dependent manner, thereby neutralizing the remifentanil stimulation of these receptors [17-19]. As a result, the use of remifentanil with sevoflurane will not aggravate slow-onset NMDA-mediated tourniquet pain and subsequent hypertension [20].
Our study has several limitations. First, because BIS is not sensitive to N2O administration when the inspired sevoflurane concentration remains high, we do not think we could adequately control the depth of anesthesia for both groups while using BIS to monitor [21]. Second, the values of EtSEVO were quite different between the groups. We acknowledge that it would have been better to standardize the concentrations of sevoflurane between groups; however, it was difficult to maintain patient vital signs and BIS within normal limits without the use of much more sevoflurane in the control group than in the remifentanil group. Finally, it is unclear whether remifentanil inhibited a hemodynamic response to tourniquet inflation via blockade of the µ receptor-mediated response. Further studies regarding this question are needed.
In conclusion, infusion with remifentanil can mitigate the hemodynamic increases associated with the use of a tourniquet in elderly patients under sevoflurane/N2O general anesthesia.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kaufman RD, Walts LF. Tourniquet-induced hypertension. Br J Anaesth. 1982;54:333-6
2. Valli H, Rosenberg PH, Kytta J, Nurminen M. Arterial hypertension associated with the use of a tourniquet with either general or regional anaesthesia. Acta Anaesthesiol Scand. 1987;31:279-83
3. Girardis M, Milesi S, Donato S, Raffaelli M, Spasiano A, Antonutto G, Pasqualucci A, Pasetto A. The hemodynamic and metabolic effects of tourniquet application during knee surgery. Anesth Analg. 2000;91:727-31
4. Kam PC, Kavanagh R, Yoong FF. The arterial tourniquet: pathophysiological consequences and anaesthetic implications. Anaesthesia. 2001;56:534-45
5. Satsumae T, Yamaguchi H, Sakaguchi M, Yasunaga T, Yamashita S, Yamamoto S, Kida H. Preoperative small-dose ketamine prevented tourniquet-induced arterial pressure increase in orthopedic patients under general anesthesia. Anesth Analg. 2001;92:1286-9
6. Yamashita S, Yamaguchi H, Hisajima Y, Ijima K, Saito K, Chiba A, Yasunaga T. Preoperative oral dextromethorphan attenuated tourniquet-induced arterial blood pressure and heart rate increases in knee cruciate ligament reconstruction patients under general anesthesia. Anesth Analg. 2004;98:994-8
7. Arai YC, Ogata J, Matsumoto Y, Yonemura H, Kido K, Uchida T, Ueda W. Preoperative stellate ganglion blockade prevents tourniquet-induced hypertension during general anesthesia. Acta Anaesthesiol Scand. 2004;48:613-8
8. Wang JY, Winship SM, Thomas SD, Gin T, Russell GN. Induction of anaesthesia in patients with coronary artery disease: a comparison between sevoflurane-remifentanil and fentanyl-etomidate. Anaesth Intensive Care. 1999;27:363-8
9. Sebel PS, Hoke JF, Westmoreland C, Hug CC Jr, Muir KT, Szlam F. Histamine concentrations and hemodynamic responses after remifentanil. Anesth Analg. 1995;80:990-3
10. Burgos LG, Ebert TJ, Asiddao C, Turner LA, Pattison CZ, Wang-Cheng R, Kampine JP. Increased intraoperative cardiovascular morbidity in diabetics with autonomic neuropathy. Anesthesiology. 1989;70:591-7
11. Turner RJ, Gatt SP, Kam PC, Ramzan I, Daley M. Administration of a crystalloid fluid preload does not prevent the decrease in arterial blood pressure after induction of anaesthesia with propofol and fentanyl. Br J Anaesth. 1998;80:737-41
12. Enoki T, Tsuchiya N, Shinomura T, Nomura R, Fukuda K. Effect of hypercapnia on arterial hypotension after induction of anaesthesia. Acta Anaesthesiol Scand. 2005;49:687-91
13. Mark JB, Steinbrook RA, Gugino LD, Maddi R, Hartwell B, Shemin R, DiSesa V, Rida WN. Continuous noninvasive monitoring of cardiac output with esophageal Doppler ultrasound during cardiac surgery. Anesth Analg. 1986;65:1013-20
14. Singer M, Clarke J, Bennett ED. Continuous hemodynamic monitoring by esophageal Doppler. Crit Care Med. 1989;17:447-52
15. Parmet JL, Horrow JC, Singer R, Berman AT, Rosenberg H. Echogenic emboli upon tourniquet release during total knee arthroplasty: pulmonary hemodynamic changes and embolic composition. Anesth Analg. 1994;79:940-5
16. Hahnenkamp K, Nollet J, Van Aken HK, Buerkle H, Halene T, Schauerte S, Hahnenkamp A, Hollmann MW, Strumper D, Durieux ME, Hoenemann CW. Remifentanil directly activates human N-methyl-D-aspartate receptors expressed in Xenopus laevis oocytes. Anesthesiology. 2004;100:1531-7
17. Orser BA, Bertlik M, Wang LY, MacDonald JF. Inhibition by propofol (2,6 di-isopropylphenol) of the N-methyl-D-aspartate subtype of glutamate receptor in cultured hippocampal neurones. Br J Pharmacol. 1995;116:1761-8
18. Criswell HE, Ming Z, Pleasant N, Griffith BL, Mueller RA, Breese GR. Macrokinetic analysis of blockade of NMDA-gated currents by substituted alcohols, alkanes and ethers. Brain Res. 2004;1015:107-13
19. Kudo M, Aono M, Lee Y, Massey G, Pearlstein RD, Warner DS. Effects of volatile anesthetics on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cultures. Anesthesiology. 2001;95:756-65
20. Fodale V, Pratico C, Tescione M, Tanania S, Lucanto T, Santamaria LB. Evidence of acute tolerance to remifentanil in intensive care but not in anesthesia. J Clin Anesth. 2006;18:293-6
21. Soto RG, Smith RA, Zaccaria AL, Miguel RV. The effect of addition of nitrous oxide to a sevoflurane anesthetic on BIS, PSI, and entropy. J Clin Monit Comput. 2006;20:145-50
Author contact
![]() Corresponding author: Jong-Man Kang, M.D., Department of Anesthesiology & Pain Medicine, Kyung Hee University Hospital at Gangdong, 149 Sangil-dong Gangdong-Gu, Seoul, Korea, Zip code: 134-090. Tel: 82-2-440-6193. Fax: 82-2-440-7808. E-mail: kjmor.kr
Corresponding author: Jong-Man Kang, M.D., Department of Anesthesiology & Pain Medicine, Kyung Hee University Hospital at Gangdong, 149 Sangil-dong Gangdong-Gu, Seoul, Korea, Zip code: 134-090. Tel: 82-2-440-6193. Fax: 82-2-440-7808. E-mail: kjmor.kr

 Global reach, higher impact
Global reach, higher impact