3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(3):237-242. doi:10.7150/ijms.4173 This issue Cite
Research Paper
COX-2 Expression and Tumor Angiogenesis in Thyroid Carcinoma Patients among Northeast Chinese Population-Result of a Single-Center Study
Department of Hepatobiliary and Pancreatic Surgery, the First Bethune Hospital, Jilin University, Jilin 130021, China.
* Bai Ji and Yahui Liu contributed equally to this paper.
Received 2012-1-31; Accepted 2012-4-15; Published 2012-4-19
Abstract
Objective: Cyclooxygenase-2 (COX-2), one of the rate-limiting enzymes in the metabolism of arachidonic acid which is reported to be involved in the pathogenesis of many human tumors. As well, Vascular endothelial growth factor (VEGF) is well known to be involved in the infiltration and metastasis of many kinds of cancers. The aim of this study was to further elucidate the clinicopathologic significance of the immunohistochemical expressions of COX-2 and VEGF in thyroid carcinoma.
Methods: Eighty-five patients with thyroid neoplasms were enrolled in our study from December 2003 to January 2010 from the authors' institution retrospectively. Their tumors were examined in the Department of Pathology, the First Bethune Hospital of Jilin University. Immunohistochemistry was performed on paraffin-embedded tissues sections using monoclonal anti-human COX-2 and VEGF antibodies. The tissues were classified into four types: papillary, follicular, medullary and undifferentiated. The patients ranged in age from 23 to 71 years. Breast cancer slides acted as control slides. The immunohistochemical stains were quantified by staining intensity and by the proportion of positively stained cells which were stained brown or yellow.
Results: The results were analysed by χ2 test. COX-2 and VEGF expressions were stronger in thyroid carcinoma than in thyroid adenomas and normal tissues (P<0.01). COX-2 and VEGF expressions in thyroid carcinoma correlated with the tumor type and TNM stage.
Conclusion: Our results suggest that expression of COX-2 and VEGF may promote angiogenesis of thyroid carcinoma, its infiltration, and metastasis.
Keywords: thyroid carcinoma, cyclooxygenase-2, angiogenesis, VEGF, northeast Chinese.
Introduction
Cyclooxygenases (COX), components of the arachidonic acid cascade are a family of catalyzing enzymes that convert cellular arachidonic acid to prostaglandin. There are two major cyclooxygenases, COX-1 and COX-21. COX-1 is a housekeeping gene that is expressed constitutively in a variety of human tissues. COX-2 is an inducible gene2. Recently it has been reported that COX-2 is overexpressed in many human tumors, including colorectal carcinoma, gastric carcinoma, gallbladder carcinoma and liver cancer3-6. This observation has suggested that COX-2 may play a critical role in the carcinogenesis and progression of tumors. Many researchs have demonstrated that COX-2 is not only responsible for cell proliferation and transformation, but also for inducing angiogenesis7,8. However, the mechanism is unclear.
Thyroid carcinoma is one of the common malignant human tumors. Its prognosis correlates with its pathological type. The prognosis of papillary carcinoma being the best, followed by follicular carcinoma and medullary carcinoma9. The prognosis of undifferentiated carcinoma is worst with a high mortality rate10. It is well known that angiogenesis plays an important role in the growth and metastasis of thyroid carcinoma4. Thyroid carcinoma cells can potentially secrete many provascular factors to promote angiogenesis. To date, vascular endothelial growth factor (VEGF) appears to be the most important angiogenic factor, and may be responsible for the infiltration and metastasis of thyroid carcinoma11,12. But the mechanism that regulats its overexpression in tumor cells is unclear. In this study we investigated the expression of COX-2 and VEGF in thyroid carcinoma, and then analyzed their correlation and the relationship between them and the pathological type and TNM stage.
Materials and methods
Patient enrollment
The study protocol was approved by the ethics committee at the First Bethune Hospital of Jilin University. Following the Institutional Guideline for informed consent in our hospital, Paraffin-embedded tissue samples were obtained from the Department of Pathology at the First Hospital of Jilin University. There were 35 male and 50 female patients, ranging in age from 23 to 71 years. The specimens included 10 normal thyroid tissues, 15 thyroid adenomas, and 60 thyroid carcinomas. According to the WHO classification criteria13, there were 28 papillary carcinomas, 10 follicular carcinomas, 12 medullary carcinomas, and 10 undifferentiated carcinomas. According to TNM (International Union Against Cancer, UICC) criteria, there were 19 stage I tumors, 23 stage II tumors, 8 stage III tumors and 10 stage IV tumors. None of the patients had been treated with radiotherapy or chemotherapy before surgery. All samples were re-examined by hematoxylin-eosin staining and diagnosis confirmed on the basis of it. The clinical records of all patients were reviewed to determine patients follow-up status and lymph node metastasis of thyroid carcinoma statistically, which referred to the postoperative pathology of dissected lymph nodes around tumors.
Antibody
SABC (Strept Avidin-Biotin Complex) immunohistochemical method and rabbit anti-human polyclonal antibodies to COX-2(3F7) and VEGF-A(SP21) were purchased from Fuzhou Maxin Biotechnical Company (Fuzhou, China). They were employed at a dilution 1:50 which was the proper concentration for the best results.
Immunohistochemistry
SABC immunohistochemical method was used to detect the expression of COX-2 and VEGF. The formalin-fixed tissues were embedded in paraffin, and sectioned at a thickness of 4μm. The sections were deparaffinized and hydrated gradually. Sections were heated in a microwave oven for 15 min to retrieve antigens. The sections were incubated with non-immune goat serum for 15 min at room temperature to eliminate non-specific staining. The sections were then incubated with the primary antibody against COX-2 and VEGF (dilution 1:50) for 60 min at room temperature, washed three times with PBS for 5min, and incubated with the secondary antibody for 15 min followed by avidin-biotin-peroxidase for 15 min at room temperature. Finally, the slides were washed three times for 15 min with PBS, visualized with DAB reagent, and counterstained with haematoxylin. Negative and positive controls were used simultaneously to ensure specificity and reliability of the staining process. The negative controls were obtained by using PBS instead of the primary antibody. Some studies have reported that COX-2 is overexpressed in breast cancer. So we chose breast cancer sections as positive controls14,15.
Immunohistochemical evaluation
Sections were observed under a light microscope after having been mounted. Positive staining with COX-2 and VEGF were defined as brown staining in the cytoplasm or nuclei. According to Kawasaki's method16, the antigen expression was quantified on the basis of staining intensity and the proportion of positively stained cells. The staining intensity of positive cells was graded as 0, 1, 2, 3. On the basis of the proportion of positive cells, slides were classified as 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), 4 (76-100%). An overall staining score was determined by the product of these two grades: 0, ≤3, 3-6 and ≥6 were defined as negative (-), positive(+), moderate positive (++), and strong positive (+++). (For each slice, five regions were evaluated at a magnification of 100. An average score was determined to avoid random sampling.)
Statistical analysis
All statistical analysis were carried out with SPSS software version 10.0. The relationship between COX-2 expression and categorical variables was compared by the χ2 test or Fisher two-sided exact probability test. Continuous variables were analyzed with student t test. A P value <0.05 was considered to be statistically significant. Correlation analysis was performed by the Spearman method.
Results
Expression of COX-2 and VEGF protein in thyroid cancer tissues
Yellow or brown granules could be seen diffusely in the cytoplasm of COX-2 positive cells (Fig. 1). Tumor cells and vascular endothelial cells with diffuse brown granules were defined as VEGF positive cells (Fig. 2). No stained granules could be seen in normal thyroid tissues. The expressions of COX-2 and VEGF in thyroid carcinomas were higher than in thyroid adenomas (P<0.01). The expression of COX-2 and VEGF in undifferentiated carcinoma was most strong and greater than in papillary or follicular carcinomas. The positive rate correlated with the TNM stage and lymph node metastasis (Table 1).
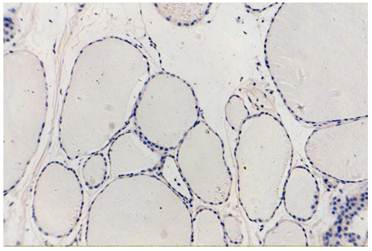
normal thyroid tissue, COX-2(-)VEGF(-), original magnification x200.
Relationship between the expression of COX-2 and VEGF and parameters of clinical pathology in thyroid cancer.
| Group | n | COX-2 | VEGF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | - | χ2 | P | + | - | χ2 | P | |||
| Normal thyroid | 10 | 0 | 0 | 0 | 0 | |||||
| Thyroid adenoma | 15 | 2(13) | 13(87) | 12.054 | 0.001a | 6(40) | 9(60) | 6.756 | 0.009e | |
| Thyroid cancer | 60 | 38( 63) | 22(37) | 45(75) | 15(25) | |||||
| Tissue type | ||||||||||
| Papillary type | 28 | 10(36) | 18(64) | 11.951 | 0.008b | 16(57) | 12(43) | 9.056 | 0.029f | |
| Follicular type | 10 | 7(70) | 3(30) | 5(50) | 5(50) | |||||
| Medullary type | 12 | 9 (75) | 3 (25) | 10(83) | 2(17) | |||||
| Undifferentiated type | 10 | 9(90) | 1(10) | 10(100) | 0(0) | |||||
| TNM stage | ||||||||||
| Ⅰ~Ⅱ | 42 | 21(50. | 21(50) | 8.061 | 0.005c | 27(64) | 15(36) | 5.860 | 0.015g | |
| Ⅲ~Ⅳ | 18 | 16(89) | 2(11) | 17(95) | 1(5) | |||||
| Lymphatic metastasis | ||||||||||
| Yes | 23 | 19(83) | 4(17) | 6.920 | 0.009d | 22(96) | 1(4) | 5.840 | 0.020h | |
| No | 37 | 18(49) | 19(51) | 24(65) | 13(34) | |||||
COX-2:
a;Thyroid adenoma vs Thyroid cancer: χ2=12.054, P=0.001;
b:Papillary type vs Follicular type vs Medullary type vs Undifferentiated type: χ2=11.951, P=0.008;
c;Stage I-II vs Stage III-IV: χ2=8.061, P=0.005;
d:Lymphatic metastasis vs No lymphatic metastasis: χ2=6.920, P=0.009;
VEGF:
e:Thyroid adenoma vs Thyroid cancer: χ2=6.756, P=0.009;
f:Papillary type vs Follicular type vs Medullary type vs Undifferentiated type: χ2=9.056, P=0.029;
g:Stage I-II vs Stage III-IV: χ2=5.860, P=0.015;
h:Lymphatic metastasis vs No lymphatic metastasis: χ2=5.840, P=0.020.
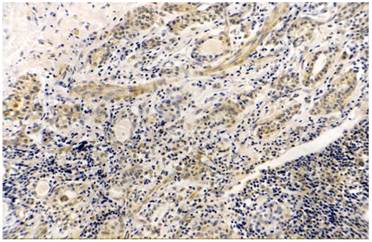
Thyroid papillary cancer, COX-2 (+++), original magnification x200.
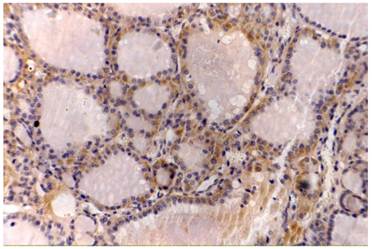
Thyroid papillary cancer, VEGF (++), original magnification x200.
Correlation between COX-2 and VEGF expressions in thyroid cancer tissues
Among the 38 COX-2 positive cases, 36 cases were associated with positive VEGF staining. The positive rate of VEGF in the COX-2 positive group was much higher than that in the COX-2 negative group. Among 15 VEGF negative cases, 13 cases presented with negative COX-2 staining. The difference between the two groups was statistically significant. COX-2 and VEGF expression demonstrated a tendency towards having a positive correlation with a correlation coefficient of 0.59 (P<0.01) determined by the Spearman method (Table 2).
Discussion
It has been documented that COX-2 plays an important role in the development of human tumors and suggested that COX-2 may promote the tumor growth and invasion17-19. Recent research has found that COX-2 can be detected in many human tumors and may be overexpressed in tumor tissues compared with normal tissues. Uefuji et al20 investigated the expression of COX-2 in 23 cases of gastric adenocarcinoma, and found in 19 cases (83%) that COX-2 expression was positive by immunohistochemical staining. In addition, Eberhart21 examined 14 cases of human colon cancer and found increased levels of COX-2 mRNA in 12 cases (86%). In colonic adenomas the expression rate of COX-2 mRNA was 43% (6/14). Tucker et al22 discovered that the level of COX-2 mRNA in pancreatic cancer was 60 times more than in adjacent cancerous tissues. Molina et al5 found COX-2mRNA amplification in 21 cases of pancreatic cancer. Among them, in 67% cancer tissues COX-2 immunohistochemistry staining was positive. This observation suggest that overexpression of COX-2 is a characteristic of malignant tumors.
Relationship between the expression of COX-2 and VEGF in thyroid cancer.
| VEGF | COX-2 | ||
|---|---|---|---|
| - | +-++ | +++ | |
| — | 13# | 1 | 1 |
| +-++ | 6 | 12 | 3 |
| +++ | 3 | 5 | 16* |
#P<0.01 compared with COX-2(+++);*P<0.01 compared with COX-2(-); r=0.636.
To the best of our knowledge, only a small number of studies have examined COX-2 expression in thyroid carcinoma23. However, their research did not investigate possible correlation between COX-2 and VEGF. Their conclusions about the clinicopathologic correlation of COX-2 expression in thyroid carcinoma remain controversial. In this study, we found that the expression of COX-2 and VEGF were much higher in thyroid carcinomas than in thyroid adenomas and normal thyroid tissues. Especially, their expression in thyroid cancer correlated with the pathologic type, clinical period and tumor stage. The expressions of COX-2 and VEGF in undifferentiated carcinomas and medullary carcinomas were higher than in papillary and follicular carcinoma, and the differences were significant statistically (P<0.01). It is known that compared with papillary carcinoma the prognosis of undifferentiated carcinoma and medullary carcinoma is worse10. Our data confirm that overexpression of COX-2 correlates with the pathologic type of thyroid carcinoma, and suggest that COX-2 overexpression is associated with a poor prognosis. In addition,the expression of COX-2 in thyroid adenomas was higher than that in normal tissues. These findings suggest that overexpression of COX-2 may be an early event in the progression of thyroid neoplasms.
It is recognized that the growth of tumors requires adequate blood to supply nutrition and oxygen. Consequently, the ability of tumors to induce angiogenesis is very important for the tumor growth24-26. Tsujii et al27 have demonstrated that COX-2 may promote carcinogenesis by a paracrine mechanism. They co-cultured endothelial cells and colon cancer cell,and discovered that COX-2 could induce the colon cancer cells to release vasculogenic prostaglandin which promoted endothelial migration and angiogenesis. This process could be inhibited by aspirin, NS-398 (a selective inhibitor of COX-2), and antibodies to provascular factors. Uefuji et al20 investigated the expression of COX-2 in 42 cases of primary gastric adenocarcinoma. They found microvessel density in tissues overexpressing COX-2 was much higher than that in tissues with low COX-2 expression (P<0.01). Animal experiments have also confirmed that the selective COX-2 inhibitor Celecoxib can inhibit angiogenesis of transplanted rat lung and colon tumors2. Hence, a further investigation is required regarding the clinical use of COX-2 inhibitors as a possible therapeutic strategy for thyroid carcinoma, especially tumors with a strong expression of COX-2. On the basis of these studies, we suggest that the mechanism by which COX-2 induces angiogenesis correlates with the expression of provascular factors.
In our study, VEGF was also overexpressed in all types of thyroid carcinoma, and it correlated with the degree of differentiation and tumor stage. It has also been demonstrated that VEGF plays a critical role in the pathogenesis of thyroid cancer. Fu et al28 found that COX-2 inhibitors restrained tumor angiogenesis, and downregulated the expression of VEGF. This suggested that COX-2 overexpression was probably one of initiation mechanisms for VEGF overexpression. In our study, we detected co-expression of COX-2 and VEGF in thyroid cancer. COX-2 and VEGF may together be involved in the progression of thyroid cancer. We performed correlation analysis of the expression of COX-2 and VEGF in thyroid cancer, and found COX-2 and VEGF expression presented a positive correlation. This suggests that COX-2 and VEGF interact in the pathogenesis of thyroid cancer. It is suggested that COX-2 may be involved in the transcriptional regulation of VEGF gene. This study provides further understanding of the molecular mechanism of thyroid carcinogenesis and adds experimental evidence for a relationship between COX-2 expression and carcinogenesis.
In summary, there was a positive correlation between COX-2 and VEGF expressions. This provided further weight to the suggestion to the motion that COX-2 may promote carcinogenesis by inducing angiogenesis29. This is consistent with previous research results. It suggests that an antisense DNA technique or COX-2 inhibitors might interfere with the expression of COX-2 or inhibit its activity to restrain the expression of VEGF and inhibit carcinogenesis30,31.
Conclusion
In our study, we found that COX-2 and VEGF expression in thyroid cancer was higher than in benign thyroid adenomas and normal thyroid tissues. In addition, our research shows there is a positive correlation between COX-2 and VEGF expressions. This indicates that COX-2 may promote carcinogenesis by inducing angiogenesis. This is consistent with previous research results. It suggests that an antisense DNA technique or COX-2 inhibitors might interfere with the expression of COX-2 or inhibit its activity to silence expression of VEGF and inhibit carcinogenesis. Hence, as an early event of carcinogenesis, COX-2 might be a potential gene therapy target, worthy of further research.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Smith WL, Meade EA, DeWitt DL. et al. Interaction of PGH synthase isozymes-1 and -2 with NSAIDs. Ann N Y Acad Sci. 1994;744:50-7
2. Masferrer JL, Leahy KM, Koki AT. et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-11
3. Fujita H, Koshida K, Keller ET. et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53:232-40
4. Krishnan J, Kirkin V, Steffen A. et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713-22
5. Molina MA, Sitja-Arnau M, Lemoine MG. et al. Increased COX-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by NSAIDs. Cancer Res. 1999;59:4356-62
6. Talar-Wojnarowska R, Gasiorowska A, Olakowski M. et al. Role of cyclooxygenase-2 gene polymorphisms in pancreatic carcinogenesis. World J Gastroenterol. 2011;17:4113-7
7. Leung WK, To KF, Go MY. et al. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol. 2003;23:1317-22
8. Lee NO, Park JW, Lee JA. et al. Dual action of a selective cyclooxygenase-2 inhibitor on vascular endothelial growth factor expression in human hepatocellular carcinoma cells: novel involvement of discoidin domain receptor 2. J Cancer Res Clin Oncol. 2012;138:73-84
9. Ladurner D, Seeber G, Hofstädter F. Papillary thyroid cancer--prognosis and prognostic factors. Langenbecks Arch Chir. 1984;363:43-55
10. Staunton MD, Skeet RG. Thyroid cancer: prognosis in 469 patients. Br J Surg. 1979;66:643-7
11. Masunaga R, Kohno H, Dhar DK. et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064-8
12. Tomozawa S, Tsuno NH, Sunami E. et al. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer. 2000;83:324-8
13. Dobert N, Menzel C, Oeschger S. et al. Differentiated thyroid carcinoma: the new UICC 6th edition TNM classification system in a retrospective analysis of 169 patients. Thyroid. 2004;14:65-70
14. Cho MH, Yoon JH, Jaegal YJ. et al. Expression of cyclooxygenase-2 in breast carcinogenesis and its relation to HER-2/neu and p53 protein expression in invasive ductal carcinoma. Breast. 2006;15:390-8
15. Surowiak P, Materna V, Matkowski R. et al. Relationship between the expression of cyclooxygenase 2 and MDR1/P-glycoprotein in invasive breast cancers and their prognostic significance. Breast Cancer Res. 2005;7:862-70
16. Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by surviving predicts shorter survival rates in colorectal cancer. Cancer Res. 1998;58:5071-4
17. Cianchi F, Cortesini C, Bechi P. et al. Up-regulation of cyclooxygenase 2.gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339-47
18. Peng ZH, Wan DS, Li LR. et al. Expression of COX-2, MMP-2 and VEGF in stage II and III colorectal cancer and the clinical significance. Hepatogastroenterology. 2011;58:369-76
19. Yao M, Kargman S, Lam EC. et al. Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res. 2003;63:586-92
20. Uefuji K, Ichikura T, Mochizuki H. et al. Expression of COX-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168-72
21. Eberhart CE, Coffey RJ, Radhika A. et al. Up-regulation of Cyclooxygenase-2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-8
22. Tucker ON, Dannenberg AJ, Yang EK. et al. Cyclooxygenase-2 expression is upregulated in human pancreatic cancer. Cancer Res. 1999;59:987-90
23. Specht MC, Tucker ON, Hocever M. et al. Cyclooxygenase-2 expression in thyroid nodules. The Journal of Clinical Endocrinology & Metabolism. 2002;87:358-63
24. Furudoi A, Tanaka S, Haruma K. et al. Clinical significance of vascular endothelial growth factor C expression and angiogenesis at the deepest invasive site of advanced colorectal carcinoma. Oncology. 2002;62:157-66
25. Kawamori T, Rao CV, Seibert K. et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409-12
26. Leahy KM, Ornberg RL, Wang Y. et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625-31
27. Tsujii M, Kawano S, Tsuji S. et al. Cyclooxygenase regulation angiogenesis induced by colon cancer cells. Cell. 1998;93:705-16
28. Fu SL, Wu YL, Zhang YP. et al. Anti-cancer effects of COX-2 inhibitors and their correlation with angiogenesis and invasion in gastric cancer. World J Gastroenterol. 2004;10:1971-4
29. Grösch S, Tegeder I, Niederberger E. et al. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742-4
30. Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmaco Sci. 2003;24:96-102
31. Kim YY, Lee EJ, Kim YK. et al. Anti-cancer effects of celecoxib in head and neck carcinoma. Mol Cells. 2010;29:185-94
Author contact
![]() Corresponding author: Guangyi Wang, MD, Department of Hepatobiliary and Pancreatic Surgery, the First Bethune Hospital, Jilin University, Jilin 130021, China (Tel: 86-431-88783331; Email:wgymdcom).
Corresponding author: Guangyi Wang, MD, Department of Hepatobiliary and Pancreatic Surgery, the First Bethune Hospital, Jilin University, Jilin 130021, China (Tel: 86-431-88783331; Email:wgymdcom).

 Global reach, higher impact
Global reach, higher impact