3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(3):207-212. doi:10.7150/ijms.3690 This issue Cite
Research Paper
Congenital Bronchial Atresia: Diagnosis and Treatment
Department of Thoracic Surgery, General Hospital of the People's Liberation Army, Beijing 100853, China
# Contributed equally.
Received 2011-10-22; Accepted 2012-2-22; Published 2012-3-3
Abstract
This study aimed to retrospectively summarize the clinical signs, diagnosis, and treatment of congenital bronchial atresia (CBA) in 12 patients. Chest radiographs and computed tomographic (CT) images of 12 patients with CBA treated in the Chinese People's Liberation Army General Hospital were reviewed. Analysis of chest radiographs revealed ten patients had hilar mass-like shadows and two had pneumonia-like shadows; most patients (n = 8) showed hyperlucency of the peripheral lung fields. CT revealed a mucocele in all the patients (n = 12); the mucoceles were round in four patients and club-like in eight. In 80% of the cases (n = 10), associated anomalies, including occlusions of the bronchus central to the mucocele, emphysematous changes of the peripheral lung fields, bronchogenic cyst, and anomalous branching of the bronchial tree and vascular structure were observed. CBA was detected in the right lobe in eight patients and the left lobe in the remaining four. No surgical intervention was performed in 5 CBA patients and the remaining 7 patients underwent surgery, including lobectomy in 5 patients and local resection in 2 patients. Among these 7 patients, 3 had a preoperative diagnosis of malignant disease, and the remaining 4 had severe clinical symptoms that could not be effectively treated by medicines. All patients were followed up, and none experienced obvious discomfort. CBA is a relatively rare and benign malformation disease. Chest CT is the procedure of choice for diagnosis. The presence of a bronchocele and surrounding emphysematous changes are typical radiologic findings in CBA. Surgery should be reserved only for patients with serious complications secondary to the atretic bronchus.
Keywords: bronchial atresia, lung, diagnosis, surgical treatment
Introduction
Congenital bronchial atresia (CBA) is a rare congenital abnormality resulting from focal interruption of a lobar, segmental, or subsegmental bronchus with associated peripheral mucus impaction (bronchocele or mucocele) and associated hyperinflation of the obstructed lung segment. In this study, we reviewed 12 cases of CBA treated in the Chinese People's Liberation Army General Hospital and discussed the diagnosis, surgical indications, as well as treatment efficacy.
Clinical Data
Materials and methods
Medical records of 12 patients diagnosed with CBA from 1980 to 2008 at the Chinese People's Liberation Army General Hospital were retrospectively reviewed. Chest radiography and computed tomography (CT) were performed for all patients. In some cases, 3D image reconstruction and virtual navigation were performed. The diagnosis of CBA was made on the basis of CT and bronchofibroscopy or postoperative pathological findings. Patient clinical data, image examination, bronchofibroscopic findings, treatment and outcome, and pathological results were gathered and analyzed.
Results
Patient clinical data
Our study included 12 patients. This case series included three children and nine adults. Children were aged nine, ten, and 11 years; the adults were aged 25-74 years, with a mean age of 37 years. There were five male and four female patients. Seven patients were asymptomatic, and five were symptomatic. Symptoms included productive cough, hemoptysis, fever, and dyspnea.
Image examination findings
The collected imaging results are shown in Fig. 1.
A 54-year-old male with a pulmonary lesion identified on a routing chest computed tomographic scan. Axial lung window (A), axial soft-tissue window (B), and minimum-intensity-projection (MinIP) lung window coronal (C), and sagittal (D) contrast-enhanced CT images show a dilated, club-like structure surrounded by emphysematous changes of the peripheral lung fields at the superior segment of the left lower lobe; computerized tomography number is 28 HU; non-enhancement of the structure is shown on contrast-enhanced CT. The orifice of the superior segmental bronchus was not observed. CT features indicate a diagnosis of bronchial atresia. E, F, G, H are the corresponding normal control CT findings of A.B, C, D.
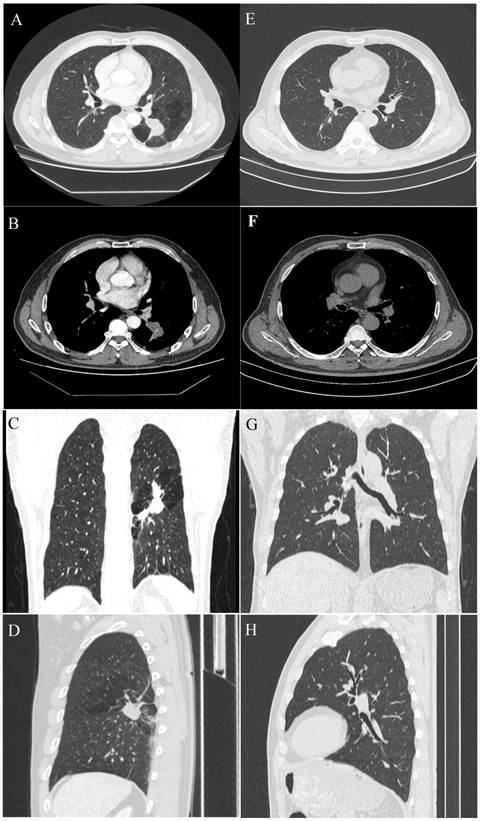
On chest radiographs, ten patients had hilar mass-like shadows, and two had pneumonia-like shadows. Most (n = 8) showed hyperlucency of the peripheral lung fields. CT revealed a mucocele in all patients (n = 12); the mucoceles were round in 4 patients and club-like in 8. In 80% of the cases (n = 10), associated anomalies, including occlusions of the bronchus central to the mucocele, emphysematous changes of the peripheral lung fields, bronchogenic cyst, and anomalous branchingof bronchial tree and vascular structure. In some cases, post-processing of CT data was conducted using 3D reconstruction, which clearly revealed the hyperinflation and a dilated bronchocele. In our study, CBA was involved the right lobe in 8 patients and the left lobe in the remaining 4.
Bronchofibroscopic diagnosis
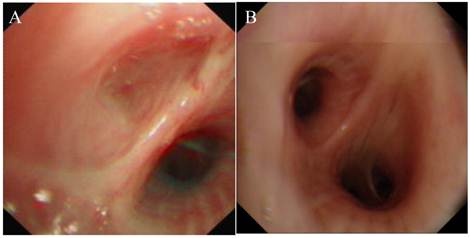
All 12 patients in our study underwent bronchofibroscopic examination. In only six cases (50%), the pathognomonic blind-ending bronchus was identified, with the location of the atretic bronchus as follows: right lung (B2b, B4, B6, B6a); left lung (B5, B8b). Three were segmental and 3 were subsegmental (Fig. 2).
Pathological results
Seven patients underwent an operation, five underwent lobectomy, and two underwent local resection. The characteristic mucocele distal to the atretic bronchus and the hyperinflated lung parenchyma were visible in all seven cases. Because the preoperative diagnosis of most CBA cases was not definite, further the distal bronchi of the operative specimens were not carefully dissected by a pathologist. The proximal blind-ending bronchus was identified in only two cases.
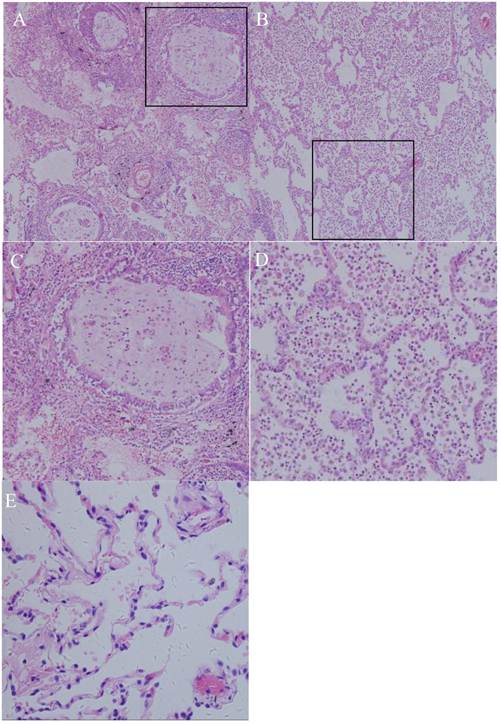
Diagnostic microscopic findings were obtained in all seven cases, with the bronchioles plugged with mucus and surrounded by distended alveoli. The septa were thin, fine, and interconnected with one another, a finding indicative of lobar hyperinflation; in some cases, inflammatory infiltrate was observed on the lung parenchyma (Fig. 3).
Diagnostic data
All 12 patients in our series underwent chest plain radiography and CT as well as bronchoscopy. Among the 12 patients in our series, three were diagnosed pathologically after operation, even though the preoperative diagnosis was a malignant disease. The remaining nine patients (75%) were diagnosed with CBA on the basis of radiological and bronchoscopic findings. In three of these patients, the operative pathologic results also confirmed the diagnosis. The others were well during the remaining years of observation.
Treatment and outcome
For treatment, no surgical intervention was required for five asymptomatic CBA patients, but follow up was required to observe the clinical course. Surgical procedures were performed in the remaining seven patients. Among these seven patients, four had serious pulmonary infections that could not be effectively controlled by medical treatment; diagnosis in the remaining three cases was uncertain, and malignant lesions could not be excluded. Procedures included lobectomy in five patients and local resection in two patients.
No serious complications were observed during the perioperative period. All patients were followed up, and none experienced obvious discomfort.
Discussion
Etiology
Bronchial atresia is a rare congenital disease typically characterized by segmental or lobar emphysema associated with or without mucoid impaction [1]. The exact cause of bronchial atresia is unknown; as the airway develops systematically, the lobar bronchi, subsegmental bronchi, and distal bronchioles appear in the 5th, 6th, and 16th weeks of fetal development, respectively. Bronchial atresia is hypothesized to occur as a focal bronchial interruption before birth. One theory is that bronchial atresia is caused by an intrauterine ischemia after the 16th week of gestation. Because other congenital lung anomalies that are known to develop earlier in embryogenesis occur with bronchial atresia, another possibility is that the lesion develops earlier, during weeks 4-6 of intrauterine development [2-5]. As reported by Schuster et al [6], the bronchial pattern is entirely normal distal to the site of stenosis, and the atresia is not a result of abnormal growth and development, but rather secondary to a traumatic event during fetal life. Similarly, Raynor et al [7] reported cases of secondary bronchial atresia caused by the presence of a mucosal flap, hypertrophy of bronchial mucosa, bronchial kinking secondary to herniation, or external compression of bronchi by abnormal vascular formation, such as patent ductus arteriosus and aneurysm of a pulmonary vein.
Bronchoscopy. A, complete obstruction of the orifice of the left B6. B, normal structure from bronchoscopic examination.
Photomicrograph (original magnification, ×100; hematoxylin-eosin staining) reveals the following: (A) the bronchioles plugged by mucus and the surrounding alveoli are dilated. Many neutrophils and macrophages were found within the bronchi and surrounding lung parenchyma, indicating acute or chronic infection. (B) Alveoli were enlarged, with a loss of alveolar walls. (C, D): structure of alveoli and mucus accumulation in the bronchioles(200X); (E) HE staining of normal lung.
Pathology
Pathological diagnosis is based on gross morphological analysis of the resected lung specimen. The proximal blind-ending bronchus is used as a diagnostic macroscopic indicator, and a characteristic mucocele typically is found just distal to the atresia point, where the adjacent lung parenchyma may appear normal or hyperinflated. This disease is sometimes misdiagnosed as emphysema, particularly when alveolar destruction is found, which has previously been described as other similar entities (e.g., congenital lobar emphysema) [8]. Riedlinger et al demonstrated that bronchial atresia and congenital cystic adenomatoid malformation nearly always coexist [9]. Both diseases may have the same etiopathogenesis caused by aberrant genetic programs or other abnormalities, with the timing of adverse influence as another possible variable. Recently, studies have suggested that bronchial atresia is one component of a number of different congenital lung abnormalities [10-11]. The timing, completeness, and level of airway obstruction during fetal development in bronchial atresia have been proposed to play an etiologic role in the varied pattern of these abnormalities.
Diagnosis and Differential Diagnosis
Because bronchial atresia is a benign disease without other serious complications, most cases do not necessitate surgery. Thus, correct diagnosis becomes particularly important.
Clinical Diagnosis
Bronchial atresia is often discovered incidentally since this benign disease is typically asymptomatic. For symptomatic patients, the most common clinical manifestation is recurrent pulmonary infections. Some authors have reported that the abnormality is more common in men than in women, and the mean age at diagnosis is 17 years [11].
Image Diagnosis
CT is the most sensitive imaging modality [3,10,12,13], and the characteristic chest CT findings of CBA include: (1) mucocele, (2) occlusion of the bronchus central to the mucocele, and (3) emphysematous changes of the peripheral lung field. If all 3 of these are present and acquired proximal bronchial obstruction by tumour, foreign body or inflammatory stricture can be excluded by fibreoptic bronchoscopy, and then accurate CBA diagnosis is confirmed. The mucocele is always a round or club-like area extending from the hilum, and in some cases, the mucocele may be located in a peripheral area. Distal hyperinflation is believed to be caused by collateral ventilation through intraalveolar pores of the Kohn, bronchoalveolar channels of Lambert, and interbronchiolar channels [14,15].
Bronchofibroscopic Diagnosis
Among the 12 CBA patients in our study, six patients (50%) were diagnosed by bronchoscopy; this finding showed that in CBA diagnosis, bronchoscopy is almost as essential as a chest CT examination. Identification of a blind-ending bronchus by fibroscopy was also helpful in CBA diagnosis [16,17].
Pathological Diagnosis
A proximal blind-ending bronchus with associated dilated distal segmental bronchi encased by hyperinflated lung parenchyma is diagnostic.
Differential Diagnosis
Differential diagnoses include vascular anomalies or other abnormalities with mucus impaction, such as bronchial cyst, pulmonary embolism, bronchiectasis, or intralobar sequestration. Contrast-enhanced CT and reconstructed CT images are useful in identification. Vascular anomalies seen on dynamic spiral CT assessment present as an enhancing tubular structure. Intralobar sequestration and bronchial cysts are very rarely associated with surrounding hyperaeration around the mass. CT results (mucocele with hyperaeration of the adjacent lung parenchyma) are considered pathognomonic by most doctors. Some publications suggest that because similar results are present for other serious disorders, such as lung cancer or bronchial adenoma, bronchoscopy can exclude these disorders and demonstrate the patency of the central bronchi, particularly in questionable cases [14-17].
Treatment
There is significant debate regarding the treatment of bronchial atresia. Some doctors prefer to perform surgery on all patients [18]. CBA treatment should be conservative, and regular chest roentgenogram during follow up should be performed. In younger patients, in whom the possibility of cancer risk is lower than that in older patients, if no serious clinical symptoms are observed, we do not recommend surgery; our point of view is contrary to that of some other doctors.The surgical indications include: the patient has recurrent and severe infection symptoms (such as pneumonias, dyspnea, cough, or hemoptysis) and medical treatment ineffective; malignant lesions cannot be excluded.
Because such benign disease often occurs in young patients, minimally invasive surgery, such as thoracoscopic surgery, is recommended. If possible, local resection should be performed as a first option, and on the basis of the intraoperative pathological results, a decision can be made regarding the need for further surgery. However, because lesions always invade the hilar region, it is very difficult to dissect the affected bronchial and pulmonary vessels, and local resection can be replaced by standard lobectomy.
Conclusions
CBA is a rare and benign malformation disease resulting from focal interruption of a lobar, segmental, or subsegmental bronchus. Most clinical symptoms are atypical. Correct diagnosis of this disease depends on the findings of chest CT. The presence of a bronchocele and surrounding emphysematous changes are the typical radiologic findings of CBA. Minimally invasive surgery should be reserved only for patients with serious complications.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Ramsay BH, Byron FX. Mucocele, congenital bronchiectasis, and bronchogenic cyst. J Thorac Surg. 1953;26(1):21-30
2. Jederlinic PJ, Sicilian LS, Baigelman W, Gaensler EA. Congenital bronchial atresia. A report of 4 cases and a review of the literature. Medicine (Baltimore). 1987;66(1):73-83
3. Kinsella D, Sissons G, Williams MP. The radiological imaging of bronchial atresia. Br J Radiol. 1992;65(776):681-685
4. Poupalou A, Varetti C, Lauron G, Steyaert H, Valla JS. Prenatal diagnosis and management of congenital bronchial stenosis or atresia: 4 cases. J Thorac Cardiovasc Surg. 2011;141(1):e11-4
5. Griffin N, Devaraj A, Goldstraw P, Bush A, Nicholson AG, Padley S. CT and histopathological correlation of congenital cystic pulmonary lesions: a common pathogenesis? Clin Radiol. 2008;63(9):995-1005
6. Schuster SR, Harris GB, Williams A, Kirkpatrick J, Reid L. Bronchial atresia: a recognizable entity in the pediatric age group. J Pediatr Surg. 1978;13(6D):682-689
7. Raynor AC, Capp MP, Sealy WC. Lobar emphysema of infancy: diagnosis, treatment, and etiological aspects-collective review. Ann Thorac Surg. 1967;4:374
8. Travis WD, Colby TV, Koss MN. et al. Congenital anomalies and pediatric disorders. In: (ed.) King DW. Atlas of nontumor pathology: non-neoplastic disorders of the lower respiratory tract. Washington, DC: American Registry of Pathology and the Armed Forces Institute of Pathology. 2002:939
9. Riedlinger WF, Vargas SO, Jennings RW, Estroff JA, Barnewolt CE, Lillehei CW, Wilson JM, Colin AA, Reid LM, Kozakewich HP. Bronchial Atresia Is Common to Extralobar Sequestration, Intralobar Sequestration, Congenital Cystic Adenomatoid Malformation, and Lobar Emphysema. Pediatr Dev Pathol. 2006;9(5):361-374
10. Gipson MG, Cummings KW, Hurth KM. Bronchial Artesia. Radiographics. 2009;29(5):1531-1535
11. Discioscio V, Feraco P, Bazzocchi A. et al. Congenital cystic adenomatoid malformation of the lung associated with bronchial atresia involving a different lobe in an adult patient: a case report. J Med Case Reports. 2010;4:164-166
12. Zhao SH, Cai ZL, Yang L. Multislice helical CT and chest radiographic findings in congenital bronchial atresis. Chin J Radiol. 2006;40(1):68-71
13. Laroia AT, Thompson BH, Laroia ST, van Beek EJ. Modern imaging of the tracheo-bronchial tree. World J Radiol. 2010;2(7):237-248
14. Matsushima H, Takayanagi N, Satoh M. et al. Congenital bronchial atresia: radiologic findings in nine patients. J Comput Assist Tomogr. 2002;26:86028641
15. Kawamoto S, Yuasa M, Tsukuda S, Heshiki A. Bronchial atresia: three-dimensional CT bronchography using volume rendering technique. Radiat Med. 2001;19(2):107-110
16. Ward S, Morcos SK. Congenital bronchial atresia - Presentationof three cases and a pictorial review. Clin Radiol. 1999;54:144-148
17. Psathakis K, Eleftheriou D, Boulas P, Mermigkis C, Tsintiris K. Congenital bronchial atresia presenting as a cavitary lesion on chest radiography: a case report. Cases J. 2009;2(1):17
18. Cappeliez S, Lenoir S, Validire P, Gossot D. Totally endoscopic lobectomy and segmentectomy for congenital bronchial atresia. Eur J Cardiothorac Surg. 2009;36(1):222-4
Author contact
![]() Corresponding author: Yuqi Wang, Tel: +8610-66938313; Fax: +8610-66938313; Email: wangyuqi301com
Corresponding author: Yuqi Wang, Tel: +8610-66938313; Fax: +8610-66938313; Email: wangyuqi301com

 Global reach, higher impact
Global reach, higher impact