3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(3):200-206. doi:10.7150/ijms.3810 This issue Cite
Case Report
Isolated Biliary Cryptococcosis Manifesting as Obstructive Jaundice in an Immunocompetent Adult
1. Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. China;
2. Department of Infectious Diseases, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. China.
Received 2011-11-16; Accepted 2012-2-16; Published 2012-3-1
Abstract
Cryptococcosis is a symptomatic fungal infection caused by Cryptococcus, which frequently occurs in patients who are immunologically compromised or chronically ill. Localized involvement of the hepatobiliary system in an immunocompetent adult is extremely rare. We report a unique case of isolated biliary cryptococcosis manifesting as obstructive jaundice and mimicking hilar cholangiocarcinoma in an immunocompetent woman. By integrating surgical and antifungal drug therapy, the disease was effectively controlled. Despite an increasing incidence of biliary malignancies, hepatobiliary surgeons and gastroenterologists must maintain a high index of suspicion for other rare possibilities of non-specific biliary inflammation.
Keywords: Biliary cryptococcosis, Obstructive jaundice, Cholangiocarcinoma, Cryptococcus neoformans, Itraconazole.
INTRODUCTION
Cryptococcosis is a symptomatic fungal infection with worldwide distribution caused by Cryptococcus, which frequently occurs in patients who are immunologically compromised or chronically ill [1, 2]. Clinical manifestations are usually confined to the central nervous system, lung and skin, whereas localized involvement of the hepatobiliary system is rare. We herein present an uncommon case of biliary cryptococcosis mimicking cholangiocarcinoma in an immunocompetent woman presenting with obstructive jaundice. With no evidence of the involvement of other organs, we established a diagnosis of isolated biliary cryptococcosis.
CASE REPORT
A 44-year-old native woman was admitted to our hospital for right upper quadrant discomfort and jaundice in July 2006. The patient had been healthy until 4 months prior to admission when upper abdominal pain of unknown origin first began; at that time, icteric eyes and skin with darkened urine were noted. She felt somewhat nauseous but did not vomit. There was no fever, cough, headache, diarrhea or other mental or physical complaints. Despite taking ursodeoxycholic acid capsules prescribed by her primary care provider for 20 days, she still felt malaise with no improvement in her symptoms. Three days prior to this hospitalization, she experienced a sudden onset of colicky pain in the stomach and back. The patient's past medical history and family history were unremarkable, and she had not been exposed to any drugs, toxins, or blood products. She had been a rural resident until marriage as an adult and resided in town. She had been in a monogamous relationship with her husband for more than 20 years and had no risk factors for HIV infection.
On physical examination, she was a well-developed woman with a body weight of 54 kg (body mass index: 20.6 kg/m2), although she had lost 10 kg over the last 4 months. Her vital signs were stable and within normal limits, and no positive physical exam findings except scleral icterus were present. Her complete blood count, coagulation factors, erythrocyte sedimentation rate, serum immunoglobulin, CEA, AFP and CA125 were normal, whereas anti-hepatitis viruses (A-E), anti-HIV and abnormal autoimmune antibodies were negative. Urine and stool tests were consistent with obstructive jaundice. The liver function tests and CA19-9 results are summarized in Table 1. Moreover, the flow cytometry lymphocyte subpopulations were normal at 49% CD3+, 21% CD4+, 23.4% CD8+, and 19.5% natural killer cells (TBNKTM, Latonia, KY). Her chest CT, gastroscopy and colonoscopy demonstrated no abnormalities, although abdominal ultrasonography revealed extra- and intrahepatic biliary dilatation. The outer CBD diameter was dilated to 16.1 mm as its maximum, but the lumen was narrowed to almost zero due to an irregular thickened wall (7.4 mm at its maximum). No findings that were consistent with bile duct calculus, liver neoplasm or cirrhosis were identified. Furthermore, an abdominal contrast-enhanced CT scan and an MRCP produced similar findings, i.e., a hilar biliary stricture with no evidence of abdominal masses or lymphadenopathy (Fig. 1). Accordingly, an ERCP was performed, which revealed an isolated irregular proximal CBD stricture with dilated intrahepatic biliary trees and no duodenal or ampullary tumors as well as negative brush cytology (Fig. 2A). After a multidisciplinary consultation with the gastroenterologists, radiologists and oncologists, a consensus diagnosis of primary sclerosing cholangitis rather than cholangiocarcinoma was made, partly due to the lack of malignant features consistent with cholangiocarcinoma in all of the medical images. Thus, the patient was transferred to the Gastroenterology Dept. and received a conventional diagnostic plan of glucocorticoid-free treatment. Unfortunately, within the first week posterior to the ERCP, her hyperbilirubinemia worsened, with TBil and DBil increasing to 203.5 μmol/L and 123.1 μmol/L, respectively. At that time, a repeat MRCP revealed a more indistinct biliary bifurcation and CHD, which was pathognomonic for malignancy (Fig. 1F). Therefore, a hepatic hilar cholangiocarcinoma (Klatskin tumor) emerged as the new presumptive diagnosis, and thus, an exploratory laparotomy was scheduled after comprehensive consent was obtained.
Intraoperatively, the liver was noted to be tough, green-brown in color, of generally normal size and without any masses or perihepatic enlarged lymph nodes. Thickened walls of the cystic duct, CHD, proximal CBD and initial part of the bilateral hepatic ducts were detected and ranged from 0.5-1.0 cm, which consequently narrowed the bile channels. Upon choledochotomy, packed gelatin-like exudates were found inside the lumen, and thus, a flexible choledochoscopy was performed, the results of which supported the above findings. The gallbladder and a strip of the morbid CBD were removed and submitted for frozen section pathological examination. Both samples demonstrated inflammatory granulomas with round refractive corpuscles based on hematoxylin-eosin staining. Therefore, the diagnosis of biliary cryptococcosis was made, and only T-tube drainage was performed.
Summary of the blood tests.
| 1st Admission | Pre-ERCP | Post-ERCP | POD1 | POD11 | 2 weeks after 1st Discharge | 6 weeks after 1st Discharge | 2nd Admission | 2nd Discharge | |
|---|---|---|---|---|---|---|---|---|---|
| WBC (/μl) | 4,900 | 5,100 | 3,000 | 9,200 | 5,200 | 5,300 | 4,200 | 4,000 | 3,800 |
| Hb (g/L) | 116 | 110 | 101 | 103 | 95 | 97 | 112 | 121 | 125 |
| ALT (IU/L) | 149 | 112 | 100 | 201 | 39 | 21 | 24 | 81 | 25 |
| AST (IU/L) | 209 | 131 | 150 | 278 | 46 | 31 | 42 | 90 | 36 |
| GGT (IU/L) | 231 | 232 | 295 | 301 | 115 | 69 | 44 | 119 | 58 |
| ALP (IU/L) | 269 | 284 | 361 | 444 | 210 | 136 | 109 | 206 | 94 |
| ALB (g/L) | 34.5 | 30.1 | 28.8 | 23.1 | 31.2 | 27.3 | 38.7 | 38.9 | 39.5 |
| TBil (μmol/L) | 99.1 | 160.7 | 203.5 | 215.5 | 123.1 | 75.2 | 18.8 | 83.6 | 22.6 |
| DBil (μmol/L) | 61.5 | 85.5 | 123.1 | 116.3 | 83.8 | 54.7 | 3.4 | 55.4 | 9.7 |
| TBA(μmol/L) | 114.2 | 99.4 | 116.0 | 36.7 | 23.1 | 16.6 | 0.6 | 120.0 | 4.0 |
| CA19-9(U/ml) | 132.3 | - | - | - | - | - | 19.2 | 15.9 | - |
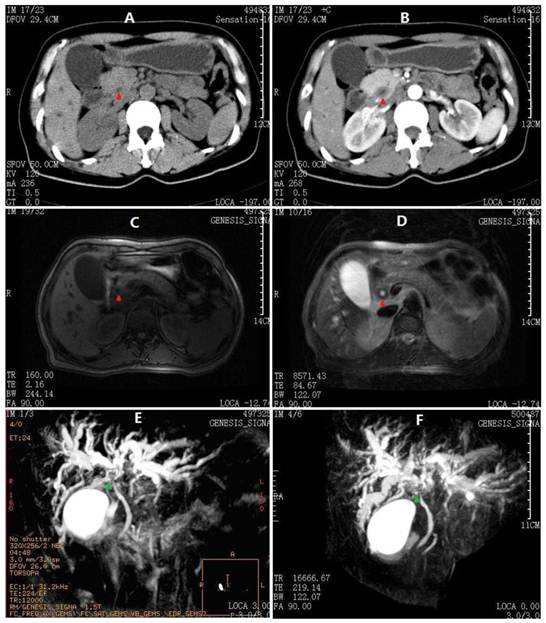
Abdominal CT and MRCP images at 1st admission. A) Thicken cystic neck, cystic duct and CBD (red arrowheads) on plain scan. B) CBD contrast enhancement in a pattern of concentric circles. C) Axial T1-W image showing moderate dilatation of intrahepatic biliary radicals in both lobes of the liver with extrahepatic bile ductal thickening. D) Axial T2-W image showing hypointense gross circumferential mural thickening of the CBD. E) The first MRCP performed before ERCP showing dilated intrahepatic ducts with abrupt cut-off of their lumens in the hilum (green arrowheads). The main pancreatic duct is not observed. F) Second MRCP after ERCP showing more dilated intrahepatic ducts with hilar discontinuity. The proximal main pancreatic duct is also observed, whereas the distal duct is not indicated.
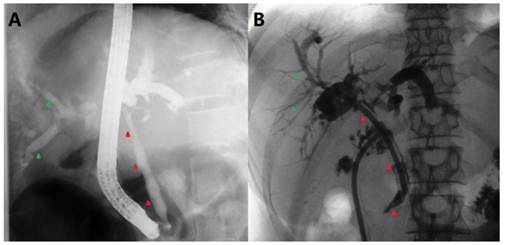
ERCP and postoperative T-tube cholangiography. A) ERCP showing an irregular CBD stricture (red arrowheads) with dilated intrahepatic biliary trees (green arrowheads). B) Intrahepatic bile duct dilatation resumed, but no definite bile duct stricture was observed.
Next, following the operation, the blood, bile, sputum, CSF, urine and stool samples were cultured simultaneously with ink staining at least in triplicate. All samples were negative for Cryptococcus, but the final paraffin pathology of the intraoperative biliary samples confirmed the definite diagnosis. Microscopic examination demonstrated numerous encapsulated yeast-like organisms measuring 7-10 μm in diameter scattered in multinucleated giant cells and fibrous tissues throughout the CBD and gallbladder wall. The periodic acid methenamine silver stain, periodic acid Schiff stain and India ink stain were all positive (Fig. 4), whereas the pathogen identified by polymerase chain reaction from the paraffin-embedded gallbladder was Cryptococcus neoformans (VNI type1) [3]. As soon as she completed an uneventful recovery, she was transferred to the Gastroenterology Dept. again for anti-fungal therapy (beginning on POD11), an itraconazole regimen consisting of 2 weeks of inpatient intravenous injections (Sporanox® IV, Xian-Janssen Pharmaceutical Ltd., Beijing, China) followed by 10 weeks of per os outpatient therapy (Sporanox® Capsule). At two weeks after discharge (i.e., 4 weeks after starting the therapy), her transaminases had returned to normal levels, whereas TBil/DBil levels normalized after an additional 4 weeks. Moreover, her T-tube was removed after a cholangiography (Fig. 2B).
During her follow-ups, she appeared to have some occasional abdominal discomfort, mainly in the right upper quadrant, but no jaundice. Regular repeat abdominal ultrasonographies revealed no extra- or intrahepatic biliary dilatation, and the thickness of CBD wall dropped to 2.2 mm (April 2009).
Unexpectedly, more than 4 years after her original presentation (September 2010), she was re-hospitalized for hyperbilirubinemia. Similar to the first admission, her examination was otherwise normal, and all body fluids were negative for Cryptococcus. The liver function test results from this presentation are listed in Table 1. Radiologic exams at this time revealed a thickened CBD wall, narrow CBD lumen and moderately dilated intrahepatic bile ducts, which were also similar to but slightly less severe than her initial presentation (Fig. 3). Instead of an ERCP, an EUS was performed; it demonstrated a benign change of her CBD entirely from the hilar bifurcation to the duodenal ampulla. A cholangioenterostomy was under deliberation; however, her icterus gradually and spontaneously vanished. By the 14th inpatient day, the patient had recovered and was again discharged. As of the time of this writing, another 16 months of follow-up have passed with no abnormalities detected.
DISCUSSION
Cryptococcus is a yeast that is present in the environment worldwide. Most species appear to be nonpathogenic to humans and animals, with the notable exceptions of Cryptococcus neoformans (formerly C. neoformans var. neoformans) and Cryptococcus gattii (formerly C. neoformans var. gattii) [3]. Clinically, cryptococcosis is known as an uncommon fungal infection that primarily affects patients with impaired T cell-mediated immunity, such as the following: patients with liver cirrhosis, systemic lupus erythematosus, diabetes mellitus, or hematologic malignancies; patients with a history of systemic corticosteroid therapy, immunosuppressive treatments, solid organ transplantation or splenectomy; and HIV-positive patients [1, 2]. The lung and central nervous system are the two most commonly affected sites, followed by the skin, prostate, eyes, adrenal glands and medullary cavity of the bones [4]. Cryptococcal involvement of the biliary system within the context of disseminated cryptococcosis in HIV-negative patients has occasionally been reported [5-11]. However, isolated biliary involvement in an otherwise normal patient as an initial presentation is exceedingly rare [12, 13].
In the present case, our assumption of “isolated biliary cryptococcosis” is reasonable, although there was no definitive autopsy evidence as provided by Bucuvalas et al [12]. First, the patient was an apparently healthy adult before and after she developed this illness, who runs her family firm competently and lives a happy personal life. During the 6-year period of clinical surveillance, no signs of impaired immunity have been identified. Second, neither clinical nor radiological infection findings in the central nervous system or lungs were noted. The Cryptococcus-negative bile, sputum, blood, CSF and stools exclude the possibility of disseminated mycosis. The thorough exploration at the time of laparotomy and the repeat medical imaging confirm the sole involvement of the biliary system. In theory, primary biliary cryptococcosis seems to be unlikely; however, this does occur in the rare instances in which implantation of the fungus is introduced directly into the bile duct [6]. Secondary biliary cryptococcosis can be a downstream sequela to liver or disseminated visceral cryptococcosis due to cryptococcemia in the systematic circulation or portal system, which is termed "the descending route".
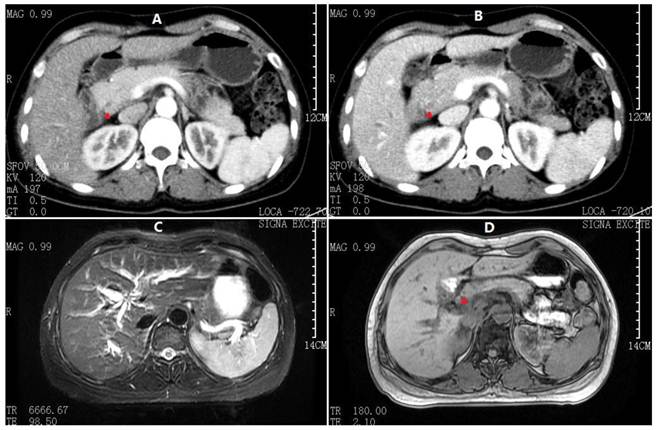
Abdominal CT and MRCP at 2nd admission. A) Arterial phase and B) Venous phase CT scan demonstrating apparently normal intrahepatic and extrahepatic bile ducts (red arrowheads denoting CBD). C) Fast-echo MRI hydrography demonstrating slight dilatation of the intrahepatic bile duct. D) Compared to Fig. 1C, the axial T1-W image shows no clear extrahepatic bile duct thickening.
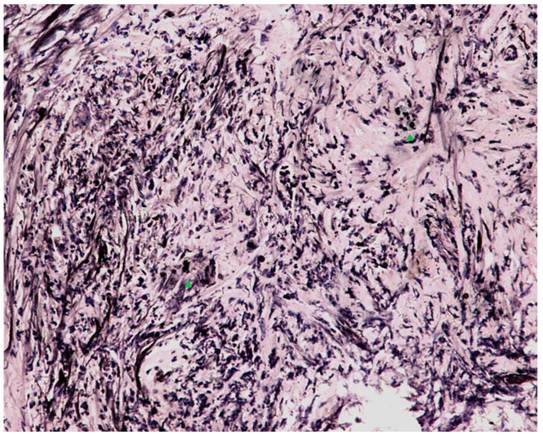
Microscopic examination with India ink staining (400x magnification). Numerous encapsulated yeast-like organisms measuring 7-10 μm in diameter were scattered in multinucleated giant cells (green arrowheads) and fibrous tissue throughout the entire CBD.
Simultaneously, intestinal inhabitation of Cryptococcus may be transmitted by "ascending" infection of the biliary tree [12, 14]. In our case, the route of infection is quite uncertain, as neither primary lesions, lymphadenectasis, nor hepatosplenomegaly are present. On the other hand, the human body in its role as the defender of infection can typically mount adequate host defenses to this organism by virtue [15]. Thus, clinically apparent cases of cryptococcosis in healthy hosts are rare, although unintentional exposure is most likely common. Evidence also indicates that cryptococcosis usually manifests as a reactivation of a dormant infection originating from significant environmental exposure during childhood when repeated inquination may be necessary to establish a latent fungal burden that can be reactivated in adulthood [16]. In summary, the etiology of our case is suspected to be a dormant ascending cholangitis due in part to the patient's rural upbringing. Furthermore, using molecular medicine techniques, we identified the exact type of Cryptococcus that was involved in this case, C. neoformans (VNI type). Although C. gattii is the species that is principally responsible for infections in immunocompetent hosts, it is rarely reported in China because it is geographically restricted to countries with tropical and subtropical climates and the temperate climate of North America [3].
From the perspective of preoperative diagnosis, it is relatively difficult to reach a precise decision. The clinical manifestations are almost not specific, presenting an unknown cause of biliary wall thickening and obstructive jaundice. Radiologically in this case, the thickened CBD exhibits a contrast enhancement in a pattern of concentric circles on the CT scans, and shows a hypointense gross circumferential mural on the axial T2-W MRI images; these may be the characteristic features of biliary cryptococcosis, according to past experiences from the systemic cryptococcosis with hepatobiliary involvement [8-13]. However, an important difference between this case and the disseminated cryptococcosis is the absence of lymphadenectasis, which just verifies the circumscription within the biliary system. The main differential diagnoses are listed in Table 2. In terms of treatments, a surgical intervention was necessary for this patient, as it was impractical to obtain reliable pathological data on bile ducts except during surgery. Analogously, biliary obstructive lesions should be treated as cholangiocarcinomas, whenever a malignancy is not definitely excluded [17].
The choice of antifungal agents depends on the site of infection and the immune status of the patient. These agents include the amphotericin B, azoles (e.g., fluconazole, itraconazole), and flucytosine. Antifungal therapy should be initiated as early as possible, as delays in antifungal therapy are associated with poorer clinical outcomes including increased mortality [18]. The recommended initial standard treatment for cryptococcal meningitis in HIV-infected adults is the amphotericin B combined with flucytosine administered over at least a 2-week induction period followed by fluconazole for 8 weeks. Itraconazole is considered an acceptable but less effective alternative. Given the hyperbilirubinemia in our patient, we selected itraconazole over amphotericin B because of the latter's slighter higher hepatotoxicity, and this choice proved to be satisfactory.
In summary, we present a unique case of isolated biliary cryptococcosis in an immunocompetent woman. Despite an increasing incidence of biliary malignancies, hepatobiliary surgeons and gastroenterologists must have a high degree of suspicion for other rare causes of non-specific biliary inflammation, thus avoid unnecessary operations for such cases.
The differential diagnosis of conditions causing thickened biliary walls and jaundice.
| Benign bile duct tumors | Benign cholangiopathy | Infectious diseases | Malignancy |
|---|---|---|---|
| papilloma | primary sclerosing cholangitis | bacterial cholangitis | lymphoma |
| adenoma | eosinophilic cholangiopathy | biliary tract stone disease | carcinoma |
| fibroma | follicular cholangitis | hepatobiliary tuberculosis | |
| neurinoma | iatrogenic bile duct stricture | parasitic cholangitis | |
| leiomyoma | ischemic cholangiopathy | clonorchiasis sinensis | |
| hamartoma | ascariasis | ||
| granular cell myoblastoma | schistosomiasis | ||
| adenomyoma | fluke | ||
| carcinoid |
Abbreviations
AFP, alpha fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CA125 (19-9), carbohydrate antigen 125 (19-9); CBD, common bile duct; CEA, carcinoembryonic antigen; CHD, common hepatic duct; CSF, cerebrospinal fluid; CT, computed tomography; DBil, direct serum bilirubin; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasonography; GGT, γ-glutamyltransferase; HIV, human immunodeficiency virus; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging; POD, postoperative day(s); TBA, total bile acid; TBil, total serum bilirubin.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Perfect JR. Cryptococcus neoformans. In: (ed.) Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases; 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier. 2010:3287-304
2. Casadevall A. Cryptococcosis. In: (ed.) Fauci AS, Braunwald E, Kasper DL. et al. Harrison's principles of internal medicine; 17th ed. New York: McGraw-Hill Medical Publishing Division. 2008:1251-3
3. Feng X, Yao Z, Ren D. et al. Genotype and mating type analysis of Cryptococcus neoformans and Cryptococcus gattii isolates from China that mainly originated from non-HIV-infected patients. FEMS Yeast Res. 2008;8:930-8
4. Kronstad JW, Attarian R, Cadieux B. et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9:193-203
5. Lefton HB, Farmer RG, Buchwald R. et al. Cryptococcal hepatitis mimicking primary sclerosing cholangitis. A case report. Gastroenterology. 1974;67:511-5
6. Symmers WS. Primary hepatic cryptococcosis. Br Med J (Clin Res Ed). 1983;287:909
7. Lin JI, Kabir MA, Tseng HC. et al. Hepatobiliary dysfunction as the initial manifestation of disseminated cryptococcosis. J Clin Gastroenterol. 1999;28:273-5
8. Kothari AA, Kothari KA. Hepatobiliary dysfunction as initial manifestation of disseminated cryptococcosis. Indian J Gastroenterol. 2004;23:145-6
9. Das CJ, Pangtey GS, Hari S. et al. Biliary cryptococcosis in a child: MR imaging findings. Pediatr Radiol. 2006;36:877-80
10. Nara S, Sano T, Ojima H. et al. Liver cryptococcosis manifesting as obstructive jaundice in a young immunocompetent man: report of a case. Surg Today. 2008;38:271-4
11. Hameed H, Sultan F, Khan YI. et al. Image of the quarter: An unusual cause of obstructive jaundice. J R Coll Physicians Edinb. 2009;39:221-3
12. Bucuvalas JC, Bove KE, Kaufman RA. et al. Cholangitis associated with Cryptococcus neoformans. Gastroenterology. 1985;88:1055-9
13. Kim JS, Choi BI, Han MC. Cryptococcal cholangiohepatitis with intraductal cryptococcoma. AJR Am J Roentgenol. 1994;163:995-6
14. Liu PY, Yang Y, Shi ZY. Cryptococcal liver abscess: a case report of successful treatment with amphotericin-B and literature review. Jpn J Infect Dis. 2009;62:59-60
15. Wozniak KL, Young ML, Wormley FL Jr. Protective immunity against experimental pulmonary cryptococcosis in T cell-depleted mice. Clin Vaccine Immunol. 2011;18:717-23
16. Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryot Cell. 2010;9:835-46
17. Davidson BR, Gurusamy K. Is preoperative histological diagnosis necessary for cholangiocarcinoma? HPB (Oxford). 2008;10:94-7
18. Perfect JR, Dismukes WE, Dromer F. et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2010;50:291-322
Footnotes
Identified by the Medical Mycology Laboratory, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University in February 2012. The materials and methods are detailed in Ref. [3].
Author contact
![]() Corresponding author: Xiao Liang, Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University; No. 3, East Qingchun Road, Hangzhou, 310016, P.R. China. E-mail: liukunedu.cn. Tel: +86-571-86006272. Fax: +86-571-86006272.
Corresponding author: Xiao Liang, Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University; No. 3, East Qingchun Road, Hangzhou, 310016, P.R. China. E-mail: liukunedu.cn. Tel: +86-571-86006272. Fax: +86-571-86006272.

 Global reach, higher impact
Global reach, higher impact