3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(1):108-114. doi:10.7150/ijms.9.108 This issue Cite
Research Paper
P-wave Dispersion for Predicting Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke
1. Selcuk University, Meram School of Medicine, Cardiology Department, Meram, Konya, 42080, TURKEY.
2. Selcuk University, Meram School of Medicine, Neurology Department, Meram, Konya, 42080, TURKEY.
Received 2011-10-18; Accepted 2011-12-8; Published 2011-12-17
Abstract
Background: Detection of paroxysmal atrial fibrillation (PAF) in acute ischemic stroke patients poses diagnostic challenge. The aim of this study was to predict the presence of PAF by means of 12-lead ECG in patients with acute ischemic stroke. Our hypothesis was that P-wave dispersion (Pd) might be a useful marker in predicting PAF in patients with acute ischemic stroke.
Methods: 12-lead resting ECGs, 24-hour Holter recordings and echocardiograms of 400 patients were analyzed retrospectively. PAF was detected in 40 patients on 24-hour Holter monitoring. Forty out of 360 age and gender matched patients without PAF were randomly chosen and assigned as the control group. Demographics, P-wave characteristics and echocardiographic findings of the patients with and without PAF were compared.
Results: Maximum P-wave duration (p=0.002), Pd (p<0.001) and left atrium diameter (p=0.04) were significantly higher in patients with PAF when compared to patients without PAF. However, in binary logistic regression analysis Pd was the only independent predictor of PAF. The cut-off value of Pd for the detection of PAF was 57.5 milliseconds (msc). Area under the curve was 0.80 (p<0.001). On a single 12-lead ECG, a value higher than 57.5 msc predicted the presence of PAF with a sensitivity of 80% and a specificity of 73%.
Conclusion: Pd on a single 12-lead ECG obtained within 24 hours of an acute ischemic stroke might help to predict PAF and reduce the risk of recurrent strokes.
Keywords: P-wave dispersion, acute ischemic stroke, 12-lead ECG, paroxysmal atrial fibrillation, 24- hour Holter monitoring.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia and constitutes the etiology in almost 15-20% of all ischemic strokes [1]. However when AF is paroxysmal a detailed investigation is warranted. Guidelines recommend at least 24-hour inpatient Holter monitoring in ischemic stroke patients albeit recent studies suggest that more prolonged monitoring might be essential to increase the chance of detecting PAF [1-3].
In previous studies, echocardiographic left atrial diameter (LAD) and premature atrial contractions on Holter monitoring were the predictors of PAF in patients with acute ischemic stroke [4, 5]. But because of high costs and technical inconvenience, standard 12-lead ECG is still the most commonly used technique [6]. P-wave dispersion (Pd) measured from a single ECG is regarded as an electrocardiographic marker of inhomogeneous and discontinuous propagation of sinus impulses [7, 8]. To the best of our knowledge, Pd has not been investigated in patients with acute ischemic stroke in whom the risk of a recurrent disabling or mortal stroke is substantially high. We aimed to determine the predictive value of Pd in this particular patient population.
Methods
This retrospective study was conducted in Cardiology and Neurology Departments of Selcuk University, Meram School of Medicine with the approval of the Institutional Ethics Committee.
Patient population
Twenty-four hour Holter records of 400 patients investigated for an acute ischemic stroke in neurology department were reviewed. Patients evaluated by a cardiologist and underwent standard 12-lead ECG recording within the first 24 hours of stroke and those were further evaluated with 24-hour Holter recordings (Pathfinder Digital Holter Analyzer and Lifecard CF Holter Recorders, Reynolds Medical, Hertford, UK) and echocardiography (Philips, Envisor C, Bothell, WA, USA) during the hospitalization period were eligible for the study. The decision of monitoring a patient with 24-hour Holter was based on the preference of attending cardiologist. All of the Holter monitorizations and echocardiograms were conducted within 2 to 7 days of admission. Acute ischemic stroke diagnosis was made by a neurologist. Acute ischemic stroke was defined as persistent neurologic deficits lasting longer than 24 hours and infarct on cranial MRI. PAF was considered when normal sinus rhythm was replaced by irregular tachycardia lasting more than 30 seconds with no visible P-wave or with unorganized F wavelets of AF on 24-hour Holter recording. Frequent atrial premature complexes (APCs) were defined as presence of ≥70 APCs per 24 hours [9]. Among 400 Holter records reviewed, PAF was diagnosed in 40 patients. Then, among remaining 360 patients without PAF on 24-hour Holter monitoring, another 40 subjects were randomly matched for age and gender and assigned as the control group.
Patients with evidence of hemorrhagic stroke, subarachnoid hemorrhage, recent myocardial infarction (<40 days), severe left ventricular systolic dysfunction (EF < 30%), decompensated heart failure, ventricular aneurysm, intracardiac thrombus or any other cause of cardiac embolism detected by transthoracic echocardiography were excluded from the study. Other exclusion criteria were previously documented AF, having rhythm other than sinus, obstructive sleep apnea, electrolyte disturbance, thyroid and pulmonary disease. The patients with transient ischemic attack were not included in the study, since accurate diagnosis of this entity consists of two challenging steps -documentation of the brief episode of neurological dysfunction and exclusion of acute infarction by neuroimaging. As endorsed by the scientific statement from American Heart Association and American Stroke Association, TIA is now regarded as “a brief episode of neurological dysfunction caused by focal brain or retinal ischemia, with clinical symptoms typically lasting less than one hour, and without evidence of acute infarction” [10]. Hence, we preferred to include only infarcts in which of all the diagnosis was confirmed by means of neurological examination findings and magnetic resonance imaging.
Analyses of ECG and echocardiographic parameters
Twelve-lead resting ECGs which were obtained in the first 24 hours of admission to the neurology department for an acute ischemic stroke were reviewed and analysed. All ECGs were recorded at a paper speed of 25 mm/s and a calibration of 1mV = 10 mm (Cardioline Delta 60 Plus, Remco SpA, Milan, Italy). To prevent the influence of circadian parameters on P-wave parameters, ECGs which were obtained in the morning hours (between 08.00 and 12.00) were analysed. One of the participant cardiologists (M.A.) who was blinded to clinical and Holter data made all the ECG analysis by using a magnifying glass.
Three consecutive beats were used for analysis and at least 10 leads were analyzable in all ECGs. P-wave duration was defined as the time measured from the onset to the end of the P-wave deflection. The onset of the P-wave was considered as the junction between isoelectric line and first visible upward or downward slope of the trace. The return of the trace to the isoelectric line was considered to be the end of the P-wave. Pd was defined as the difference between maximum and minimum P-wave durations (Pmax and Pmin, respectively) occurring in any of the 12 leads [7].
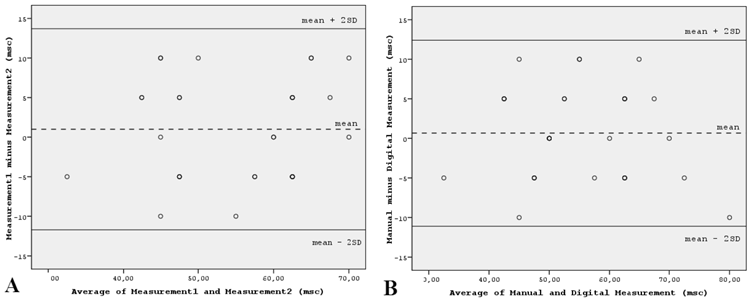
Intraobserver variability of manual Pd measurements was tested in 30 randomly selected patients. Agreement between Pd measurements was assessed using the Bland-Altman method [11]. The 95% limits of agreement for Pd were -11.7 and 13.7 milliseconds (msc), which means that there was a 95% probability that the repeated measurements differed no more than -11.7 to 13.7 msc from the first measurement. The 95% limits of agreement for Pmax were -10.6 and 11.2 msc. Furthermore, electrocardiograms of the same patient group were scanned at 300 dpi and P-wave parameters were measured by another cardiologist (U.D.) on a high resolution computer screen. When manual and digital measurements were compared, the 95% limits of agreement for Pd were -11.1 and 12.4 msc, and for Pmax were -12.1 and 13.5 msc, respectively (Figure 1).
Routine echocardiographic parameters including left ventricular ejection fraction (LV EF), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD) and LAD were also recorded. Thereafter, demographics and P-wave characteristics of the patients with and without PAF were compared.
Statistical analyses
Continuous variables were expressed as mean ± standard deviation and categorical variables as numbers. Significances of the differences between the groups were tested by the two-sided independent samples t-test. Pearson's chi-square test was used for categorical comparisons of nominal values. Simple relations between Pmax, Pd, age, heart rate, LV EF, LVEDD and LAD were assessed by using Pearson correlation. A binary logistic regression analysis was performed to identify the predictors of presence of PAF during 24-hour Holter monitoring. Receiver operating characteristic (ROC) curves were generated to identify the optimal cut-off values of Pd to predict the presence of PAF on 24-hour Holter monitoring. The validity of the model was measured by means of the area under ROC curve. A p value less than 0.05 was considered to be statistically significant. Data were analyzed by using SPSS for Windows version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
The groups of acute ischemic stroke patients with and without PAF in 24-hour Holter monitoring were well matched with regard to hypertension, diabetes mellitus, hyperlipidemia and being on medications including statins, angiotensin-converting enzyme inhibitors, angiotensin receptor, calcium channel and beta blockers. Besides, there were no significant differences between the groups regarding smoking, coronary artery disease, previous myocardial infarction, pre-existing systolic heart failure, valvular heart disease and recurrent stroke (Table 1). Heart rate, creatinine, blood urea nitrogen, total cholesterol, LDL-cholesterol and hemoglobin levels, LV end-diastolic and -systolic diameters and left ventricular ejection fraction were also similar. Proportion of patients with frequent APCs did not differ between the groups.
Maximum P-wave duration (p=0.002), Pd (p<0.001) and left atrium diameter (p=0.04) were significantly higher in patients with PAF when compared to those without PAF (Table 1). Correlation analyses between Pmax, Pd and age, heart rate, LV EF, LVEDD and LAD were performed (Table 2). A significant positive correlation was determined between Pd and LAD (β=0.36, p=0.01) and between Pmax and LAD (β=0.36, p=0.01).
Bland-Altman plots demonstrating the 95% limits of agreement between (A) the repeated measurements of Pd by the same observer and (B) between the manual and digital measurements of Pd by different observers, in 30 randomly selected patients. Abbreviations: msc: millisecond; SD: standard deviation.
Comparison of demographic characteristics, medications on admission*, echocardiographic indices, Pmax, Pmin and Pd of the patients with and without paroxysmal atrial fibrillation.
| Parameter | Patients with PAF (n=40) | Patients without PAF (n=40) | p value |
|---|---|---|---|
| Age | 69±12 | 69±13 | 0.78 |
| Gender(male/female) | 26/14 | 26/14 | 1 |
| Hypertension | 20 (50%) | 22 (55%) | 0.65 |
| Diabetes mellitus | 14 (35%) | 12 (30%) | 0.63 |
| Hyperlipidemia | 5 (12.5%) | 7 (17.5%) | 0.53 |
| Smoking | 10 (25%) | 12 (30%) | 0.62 |
| Coronary artery disease | 6 (15%) | 7 (17.5%) | 0.76 |
| Previous MI | 3 (7.5%) | 2 (5%) | 0.64 |
| Pre-existing systolic HF | 4 (10%) | 3 (7.5%) | 0.69 |
| Valvular heart disease | 1 (2.5%) | 0 (0%) | 0.31 |
| Recurrent stroke | 4 (10%) | 5 (12.5%) | 0.72 |
| Beta blocker | 5 (12.5%) | 4 (10%) | 0.72 |
| Ca blocker | 8(20%) | 7 (17.5%) | 0.78 |
| ACEI/ARB | 11 (27.5%) | 9 (22.5%) | 0.61 |
| Statin | 5 (12.5%) | 6 (15%) | 0.75 |
| LAD (mm) | 38±6 | 36±5 | 0.04 |
| LV EF (%) | 58±7 | 59±7 | 0.62 |
| LVEDD (mm) | 46±6 | 46±6 | 0.83 |
| LVESD (mm) | 27±5 | 28±6 | 0.31 |
| Heart rate (bpm) | 73±11 | 72±10 | 0.68 |
| Frequent APCs | 13 (32.5%) | 11 (27.5%) | 0.63 |
| Pmax (msc) | 115±18 | 103±13 | 0.002 |
| Pmin (msc) | 50±13 | 53±12 | 0.23 |
| Pd (msc) | 65±14 | 50±12 | <0.001 |
*Drugs which might affect the atrial conduction properties.
Abbreviations: ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; HF: heart failure; LAD: left atrial diameter; LV EF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; MI: myocardial infarction; msc: millisecond; PAF: paroxysmal atrial fibrillation; Pmax: maximum P-wave duration; Pmin: minimum P-wave duration; Pd = P-wave dispersion.
Correlations of Pd and Pmax with age, heart rate, LV EF, LVEDD and LAD in the whole patient population.
| Age | Heart rate | LV EF | LVEDD | LAD | Pmax | Pd | |
|---|---|---|---|---|---|---|---|
| Pmax | -0.05 | -0.09 | -0.08 | 0.21 | 0.36* | - | 0.69** |
| Pd | -0.02 | 0.02 | 0.07 | 0.13 | 0.36* | 0.69** | - |
Abbreviations: LAD: left atrial diameter; LV EF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter; Pmax: maximum P-wave duration; Pd: P-wave dispersion. *p < 0.05, **p < 0.01.
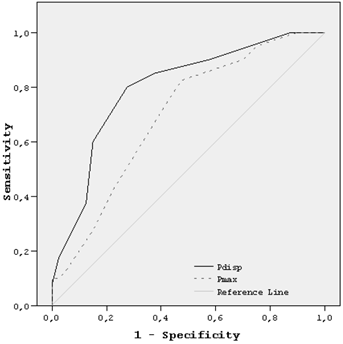
In binary logistic regression analysis, Pd was the only independent predictor of PAF in acute ischemic stroke patients (Table 3). The optimum cut-off value for Pd determined by ROC curve was 57.5 msc. On a single 12-lead ECG, a Pd value higher than 57.5 msc predicted presence of PAF with sensitivity of 80%, specificity of 73%, positive predictive value of 74% and negative predictive value of 78%. The area under curve was 0.80 (p<0.001, 95% CI: 0.70-0.90) (Figure 2).
Logistic regression analysis shows that detection of PAF on 24-hour Holter monitoring increased by odds ratio of 2.7 for each 10 msc Pd increment.
| Odds ratio | 95% CI | Sig. | |
|---|---|---|---|
| Hypertension | 0.80 | 0.25-2.64 | 0.72 |
| Diabetes mellitus | 1.20 | 0.36-4.02 | 0.77 |
| Hyperlipidemia | 0.83 | 0.17-4.19 | 0.82 |
| Smoking | 1.44 | 0.35-5.85 | 0.62 |
| Coronary artery disease | 0.35 | 0.06-2.14 | 0.25 |
| Pmax* | 1.11 | 0.68-1.83 | 0.68 |
| Pd* | 2.74 | 1.48-5.07 | 0.001 |
| LV EF (%) | 0.91 | 0.82-1.01 | 0.07 |
| LVEDD (mm) | 0.44 | 0.11-1.70 | 0.23 |
| LAD (mm) | 2.36 | 0.64-8.68 | 0.20 |
* Odds ratio per 10 msc change.
Abbreviations: LAD: left atrial diameter; LV EF: left ventricular ejection fraction; LVEDD: left ventricular end-diastolic diameter; msc: millisecond; Pmax: maximum P-wave duration; Pd: P-wave dispersion.
Receiver operator characteristic (ROC) curve demonstrating sensitivity as a function of 1-specifity for predicting presence of PAF in 24-hour ECG-Holter monitoring based on the logistic model incorporating relative contributions of Pd and Pmax. The area under the ROC curve were 0.80 (p<0.001) and 0.70 (p=0.001), respectively. Abbreviations: Pd: P-wave dispersion; Pmax: maximum P-wave duration.
Discussion
As far as we know, this is the first study demonstrating an association between Pd and PAF in patients with acute ischemic stroke. Pd is a simple index and can be easily measured by a 12-lead ECG. This might be a useful tool to predict the presence of PAF in patients with acute ischemic stroke with a sinus rhythm at the time of presentation.
Despite advances in diagnostic and interventional procedures, cardiac causes constitute the etiology in a high proportion of patients with ischemic stroke [12]. The association between PAF and stroke has been shown to be considerably strong [13]. Ischemic stroke patients -either with paroxysmal or permanent AF- have greater mortality and morbidity rates when compared to those without AF [1]. On the other hand, poor outcome associated with PAF is preventable with anticoagulation in nearly 40% of patients and it is clinically crucial to detect these patients [14]. However, unlike permanent AF, PAF might not be diagnosed readily and can be easily overlooked. Clinical guidelines recommend that patients with acute ischemic stroke should be evaluated with 24-hour inpatient monitoring to detect PAF [2]. But, 24-hour Holter monitoring might have a low yield diagnosing only 2.4% - 9.4% of these patients [3, 15, 16]. Possibility of detecting PAF with 24-hour Holter monitoring might be even lower (1-5%). Moreover, it has recently been reported that there was no significant difference between Holter monitoring and serial ECG assessment in AF detection in patients with stroke/transient ischemic attacks [17]. Similar to these data, Shafqat et al. have reported that Holter monitoring did not always detect AF in patients who were AF-positive on ECG [15]. These findings give support to the studies which suggest that Holter monitoring might have the potential of underestimating AF in ischemic stroke patients [6, 18].
The challenges mentioned above have led to investigations in this area. Pd is one of the most investigated parameters. Pd assessed on a single resting ECG is regarded as a noninvasive electrocardiographic marker which reflects the prolongation of intraatrial and interatrial conduction time in addition to discontinuous propagation of sinus impulses [7]. It has been demonstrated that prolongation of Pd is a risk factor for the development of PAF independent from the presence of structural heart diseases. It also has been shown that Pd might predict transition from PAF to permanent AF [7, 19].
In our study Pd was the only independent predictor of PAF in acute ischemic stroke patients. In logistic regression analysis, detection of PAF on 24-hour Holter monitoring increased by odds ratio of 2.7 for each 10 msc Pd increment. In ROC curve analysis Pd alone with a cut-off value of 57.5 msc had a sensitivity of 80% and a specificity of 73% to predict presence of PAF which was detected on 24-hour Holter monitoring. 95% CI of area under the curve of Pd (0.70-0.90) did not include 0.5, therefore we considered that these predictive values were reliable [20]. Normal values of Pd have ranged from 28 to 52 mseconds in the literature [21, 22]. In their study evaluating 502 adults without evident cardiovascular disease (30.3% hypertensive, 12.2% diabetic) Magnani et al. reported mean Pd value as 48±12 msc [23]. Although, mean Pd values of our study population are comparable to several studies mentioned above, they are higher than the average in the literature. This might be explained by the characteristics of our study population. Both PAF and control groups consisted of acute ischemic stroke patients with high proportions of risk factors for increased Pd such as hypertension and diabetes mellitus [24, 25].
We also found that P-wave duration (Pmax) was significantly longer in patients with PAF when compared to patients without PAF (p=0.002). This finding is consistent with previously published data in which significantly longer intraatrial and interatrial conduction time of impulses associated with P wave prolongation in 12-lead ECG recordings have been demonstrated in individuals with a clinical history of PAF [7]. However, in our study, although, Pmax had a good correlation with Pd (β=0.69, p<0.001), it was not an independent risk factor for presence of PAF. Recent literature suggests that both Pmax and Pd might be the predictors of arrhythmogenesis and nonsudden cardiac death [26]. Albeit prolonged Pmax and Pd, either separately or together, have been shown to be associated with increased incidence of AF in diverse circumstances, which one is superior to the other as a predictive risk factor remains unclear and needs to be established in large-scaled prospectively designed studies.
Bland-Altman analysis demonstrated that both intraobserver variability of repeated manual measurements and agreement between manual and digital measurements were within clinically acceptable limits. These results suggest that Pd may be considered as a reliable, inexpensive tool as a first-line diagnostic test which can alert the physician for further investigation of presence of PAF in acute ischemic stroke patients.
Consistent with previous data, there was a positive correlation between Pd and LAD which in turn might be associated with stroke due to an increased risk for thrombus formation [27]. However, association of Pd and PAF was found to be independent from LAD, since both parameters were put into logistic regression analysis.
Study limitations
Since 24-hour Holter monitoring might have a low diagnostic yield, a selection bias could have potentially influenced our results. However, it must be emphasized that, patients in which PAF is detected on 24-hour Holter monitoring still constitute a significant subgroup who are anticoagulated for secondary stroke prevention. Also, in clinical practice anticoagulant therapy is not initiated in a considerable number of patients as a consequence of absence of PAF on 24-hour Holter monitoring. Furthermore, this was a retrospective study and the decision to detect arrhythmia and to perform Holter-monitoring was based on the preference of the attending cardiologist. Data of patients who did not undergo Holter monitoring were lacking. Hence, relevance of these findings into clinical practice needs to be verified with randomized prospective studies.
Body mass index and presence of left ventricular hypertrophy are important predictors of AF [28, 29]. Additionally, left atrial volume index is reported to be superior to conventional M-mode left atrial dimension in predicting AF [30]. However, these parameters were left out due to lack of available data in medical reports of the patients.
Preexisting systolic heart failure was present in 8.8% (n=7) of all study population whereas 6.3% (n=5) had previous myocardial infarction. In their study evaluating the outcomes of pre-existing heart failure in acute ischemic stroke patients, Sharma et al. reported the prevalence of heart failure as 17% [31]. In our study, the exclusion of patients with recent myocardial infarction (<40 days), severe left ventricular systolic dysfunction (EF<30%), ventricular aneurysm, intracardiac thrombus or any other cause of cardiac embolism detected by transthoracic echocardiography might have led to lower proportions of pre-existing heart failure. Moreover, influences of seasonal and metabolic factors such as abnormal thyroid hormone levels were potential confounders which could not be excluded from the study.
Premature atrial beats are reported to be a marker for PAF in patients with acute ischemic stroke [9]. However, they were similar in both of the study groups. This result may be explained with the small sample size. Morphologic characteristics of P-wave such as the presence of electrocardiographic left atrial abnormality -which have been suggested as potential risk factors for predicting AF- could have been evaluated in addition to Pd, but were disregarded due to small sample size [32].
Conclusion
P-wave dispersion as assessed on a single 12-lead ECG might guide the physician to predict PAF in this high-risk patient population and help to reduce the risk of recurrent stroke. Due to mentioned limitations, values concerning the sensitivity and specificity must be interpreted as an attribution to the potential clinical utilization of these parameters, instead of exact values showing diagnostic accuracy. Studies based on longer monitorization of heart rhythm by external or implantable loop recorder devices would be beneficial to demonstrate definite value of P-wave parameters for predicting PAF in patients with acute ischemic stroke.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Camm AJ, Kirchhof P, Lip GY. et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369-429
2. Adams HP Jr, del Zoppo G, Alberts MJ. et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655-711
3. Liao J, Khalid Z, Scallan C, Morillo C, O'Donnell M. Noninvasive cardiac monitoring for detecting paroxysmal atrial fibrillation or flutter after acute ischemic stroke: a systematic review. Stroke. 2007;38:2935-40
4. Fujii S, Shibazaki K, Iguchi Y, Sakai K, Kimura K. [Relationship between left atrial size and paroxysmal atrial fibrillation in acute ischemic stroke]. Rinsho Shinkeigaku. 2009;49:629-33
5. Wallmann D, Tuller D, Kucher N, Fuhrer J, Arnold M, Delacretaz E. Frequent atrial premature contractions as a surrogate marker for paroxysmal atrial fibrillation in patients with acute ischaemic stroke. Heart. 2003;89:1247-8
6. Douen A, Pageau N, Medic S. Usefulness of cardiovascular investigations in stroke management: clinical relevance and economic implications. Stroke. 2007;38:1956-8
7. Dilaveris PE, Gialafos EJ, Sideris SK. et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733-8
8. Centurion OA. Clinical implications of the P wave duration and dispersion: relationship between atrial conduction defects and abnormally prolonged and fractionated atrial endocardial electrograms. Int J Cardiol. 2009;134:6-8
9. Wallmann D, Tuller D, Wustmann K. et al. Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: an opportunity for a new diagnostic strategy. Stroke. 2007;38:2292-4
10. Easton JD, Saver JL, Albers GW. et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-93
11. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-10
12. Weir NU. An update on cardioembolic stroke. Postgrad Med J. 2008;84:133-42
13. Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967-75
14. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-67
15. Shafqat S, Kelly PJ, Furie KL. Holter monitoring in the diagnosis of stroke mechanism. Intern Med J. 2004;34:305-9
16. Yu EH, Lungu C, Kanner RM, Libman RB. The use of diagnostic tests in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2009;18:178-84
17. Douen AG, Pageau N, Medic S. Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke. 2008;39:480-2
18. Rizos T, Rasch C, Jenetzky E. et al. Detection of Paroxysmal Atrial Fibrillation in Acute Stroke Patients. Cerebrovasc Dis. 2010;30:410-7
19. Koide Y, Yotsukura M, Ando H. et al. Usefulness of P-wave dispersion in standard twelve-lead electrocardiography to predict transition from paroxysmal to persistent atrial fibrillation. Am J Cardiol. 2008;102:573-7
20. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36
21. Can I, Onat AM, Aytemir K. et al. Assessment of atrial conduction in patients with scleroderma by tissue Doppler echocardiography and P wave dispersion. Cardiology. 2007;108:317-21
22. Yigit Z, Akdur H, Ersanli M, Okcun B, Guven O. The effect of exercise to P wave dispersion and its evaluation as a predictor of atrial fibrillation. Ann Noninvasive Electrocardiol. 2003;8:308-12
23. Magnani JW, Mazzini MJ, Sullivan LM, Williamson M, Ellinor PT, Benjamin EJ. P-wave indices, distribution and quality control assessment (from the Framingham Heart Study). Ann Noninvasive Electrocardiol. 2010;15:77-84
24. Dagli N, Karaca I, Yavuzkir M, Balin M, Arslan N. Are maximum P wave duration and P wave dispersion a marker of target organ damage in the hypertensive population? Clin Res Cardiol. 2008;97:98-104
25. Yazici M, Ozdemir K, Altunkeser BB. et al. The effect of diabetes mellitus on the P-wave dispersion. Circ J. 2007;71:880-3
26. Magnani JW, Gorodeski EZ, Johnson VM. et al. P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm. 2010;8:93-100
27. Tukek T, Akkaya V, Atilgan D. et al. Effect of left atrial size and function on P-wave dispersion: a study in patients with paroxysmal atrial fibrillation. Clin Cardiol. 2001;24:676-80
28. Wanahita N, Messerli FH, Bangalore S, Gami AS, Somers VK, Steinberg JS. Atrial fibrillation and obesity--results of a meta-analysis. Am Heart J. 2008;155:310-5
29. Verdecchia P, Reboldi G, Gattobigio R. et al. Atrial fibrillation in hypertension: predictors and outcome. Hypertension. 2003;41:218-23
30. Tsang TS, Barnes ME, Bailey KR. et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467-75
31. Sharma JC, Fletcher S, Vassallo M, Ross I. Cardiovascular disease and outcome of acute stroke: influence of pre-existing cardiac failure. Eur J Heart Fail. 2000;2:145-50
32. De Bacquer D, Willekens J, De Backer G. Long-term prognostic value of p-wave characteristics for the development of atrial fibrillation in subjects aged 55 to 74 years at baseline. Am J Cardiol. 2007;100:850-4
Author contact
![]() Corresponding author: Selcuk University, Meram School of Medicine, Cardiology Department, Meram, Konya, 42080, TURKEY. E-mail: umuttandogancom, Phone: +903322237506/+905335200191, Fax: +903322236181.
Corresponding author: Selcuk University, Meram School of Medicine, Cardiology Department, Meram, Konya, 42080, TURKEY. E-mail: umuttandogancom, Phone: +903322237506/+905335200191, Fax: +903322236181.

 Global reach, higher impact
Global reach, higher impact