3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(1):74-82. doi:10.7150/ijms.9.74 This issue Cite
Research Paper
Early Biventricular Molecular Responses to an Acute Myocardial Infarction
1. Department of Cardiovascular Surgery, Dokuz Eylül University, Faculty of Medicine, 35340 İnciraltı İzmir, TURKEY.
2. Department of Biomechanics, Dokuz Eylül University, Health Sciences Institute, 35340 İnciraltı İzmir, TURKEY.
3. Department of Medical Biology and Genetics, Dokuz Eylül University, Faculty of Medicine, 35340 İnciraltı İzmir, TURKEY.
4. Department of Pathology, Dokuz Eylül University, Faculty of Medicine, 35340 İnciraltı İzmir, TURKEY.
5. Department of Anesthesiology, Dokuz Eylül University, Faculty of Medicine, 35340 İnciraltı İzmir, TURKEY.
* Current address: Section for Medical Expert and Knowledge-Based Systems, Center for Medical Statistics, Informatics, and Intelligent Systems, Medical University of Vienna, Spitalgasse 23 A-1090 Vienna, AUSTRIA
Received 2011-10-21; Accepted 2011-11-16; Published 2012--
Abstract
Background: Acute myocardial infarction (AMI) remains as one of the most common lethal diseases in the world and therefore it is necessary to understand its effect on molecular basis. Genome-wide microarray analysis provides us to predict potential biomarkers and signaling pathways for this purpose.
Objectives: The aim of this study is to understand the molecular basis of the immediate right ventricular cellular response to left ventricular AMI.
Material and Methods: A rat model of left anterior descending coronary artery ligation was used to assess the effect of left ventricular AMI on both the right ventricle as a remote zone and the left ventricle as an ischemic/infarct zone. Microarray technology was applied to detect the gene expression. Gene Ontology and KEGG pathways analysis were done to identify effected pathways and related genes.
Results: We found that immune response, cell chemotaxis, inflammation, cytoskeleton organization are significantly deregulated in ischemic zone as early response within 30 min. Unexpectedly, there were several affected signaling pathways such as cell chemotaxis, regulation of endothelial cell proliferation, and regulation of caveolea regulation of anti-apoptosis, regulation of cytoskeleton organization and cell adhesion on the remote zone in the right ventricle.
Conclusion: This data demonstrates that there is an immediate molecular response in both ventricles after an AMI. Although the ischemia did not histologically involve the right ventricle; there is a clear molecular response to the infarct in the left ventricle. This provides us new insights to understand molecular mechanisms behind AMI and to find more effective drug targets.
Keywords: Acute myocardial infarction, molecular response
INTRODUCTION
Gene expression microarray technology, founded in the 1990s, which is designed to detect millions of gene targets simultaneously, has been developed for the purpose of analyzing the effects of various agents on tissue gene expression level. It has also been used to detect the gene expression in myocardial cells at infarction or remote zone samples [1, 2]. Myocardial infarction is a leading cause of cardiovascular morbidity and mortality. After infarction, a repair process is initiated that strongly depends on vascular status. Rapid angiogenesis and residual vascular potency are important to the re-establishment of coronary collateral circulation and to the improvement of blood supply to the infarction zone and its periphery. Although many studies have focused on the role of growth factors in revascularization after myocardial ischemia, specifically on the expression of growth factors and their application in therapeutic angiogenesis and on arterial blood supply through coronary revascularization, there is no knowledge about how other ventricles which is anatomically far from ischemic area integrate to this response [3-6]. This study was undertaken to delineate molecular processes underlying the response of right ventricular cells to MI after ligation of the left anterior descending coronary artery in rats. Successful MI compensation involved early remote zone gene activation those are including an acute phase response, initiation of a cytoprotective program, recruitment of extensive developmental transcription factors and induction of signaling pathways associated with cell proliferation. Despite overriding transcriptional depression, the infarct zone exhibited an early cytoprotective response through recruitment of different gene families than the remote zone [7-9].
MATERIAL AND METHOD
Animals and experimental model
Adult Wistar albino rats, weighing 250-300 g, were obtained from the Laboratory Animal Science Department, Dokuz Eylül University, and İzmir, Turkey. All experimental animal procedures were approved by Local Ethical Committee of Dokuz Eylül University Animal Care and Use. Animals were housed in controlled environmental conditions and had free access to a standard diet and tap water. Animals were maintained in controlled rooms with 12 hours light/dark cycle and a pathogen free facility. The rats were divided in 2 groups; Sham-operated rats (SHAM group), coronary artery ligation group (MI group). Sham-operated rats underwent identical surgical procedure except suture of coronary artery. The acute myocardial infraction (AMI) model was carried out by left coronary artery ligation according to the reports (Hochman and Bulkley, 1982). Rats were anesthetized with intra peritoneal ketamine (35 mg/kg) and xylazine (15 mg/kg) fixed in a supine position being shaved on the chest and then intubated with opening tracheotomy. Positive pressure respiration (10-15 ml/kg tidal volume, 60 breath/min) was started with FiO2 100% (Hugo sacs rodent ventilator, Germany). Under sterile conditions, right carotid artery exteriorizes and a catheter filled with heparinized saline was inserted into the artery, the catheter was connected through a pressure transducer to the monitor, arterial pressure monitored. Then, left intercostals' thoracotomy was performed and the third and fourth intercostals' ribs were separated with a small retractor to expose the heart. The pericardium was opened. Anterior transmural AMI was created by occlusion of the left anterior descending coronary artery with a 6-0-10 mm a traumatic proline silk suture which passed through the epicardial layer around the midway of the left anterior descending coronary artery. Following coronary occlusion, arterial blood gases, heart rate, rectal temperature and arrhythmias were recorded. After scarification and hearts were dissected at the end of thirtieth minute, tissue samples were taken out from ischemic from left ventricles and non-ischemic zones from right ventricles in both SHAM and MI groups, separately.
Histological examination
Remaining heart tissue was immersed in a buffered 10 % formalin solution and then embedded in paraffin for histopathological study. Parallel samples were then separately fixed in 10 % formal-saline, dehydrated through graded alcohol series, cleared in xylene and embedded in paraffin wax Serial sections of 5 µm thickness were cut, stained with Hematoxylin and eosin (HE) and used for the assessment of histopathological changes. Some sections were subjected to Masson's Trichrome (MT) for collagen staining. The sections were examined under light and electron microscope. Histological analysis of the infarct zone revealed that tissue integrity was abnormal including regional vacuolation and disruption of myofibrils organization with interstitial hemorrhagic foci, broadly disorganized interstitial tissue and global disruption of sarcomeric organization including contraction band necrosis.
RNA extraction and amplification
Following all operations, tissues were washed with ice cold PBS and store at -80 in RNAlatter. These tissues were snapping frozen in liquid nitrogen, pounded with mortal and pestle then homogenized with 20g syringe and qia-shredder. Total RNA from homogenized myocardial tissues was extracted by using fibrous tissue RNA isolation kit as the manufacturer's recommended protocol (Qiagen CA, USA). The quantity and purity of RNA were determined by measuring A260 and A280. Integrity of RNA was determined with 1.5% formaldehyde agarose gel electrophoresis. RNA bands were visualized with staining samples with ethidium bromide.
Gene expression microarray
10 ug of RNA was used to synthesize first and then second strand cDNA by using One-Cycle cDNA Synthesis Kit. Poly-A control RNA was amplified for intensity normalization during microarray scanning. After cleanup of second strand cDNA target labeling was performed. Biotinylated cRNA samples were prepared according to the standard Affymetrix GeneChip® protocol. Quantity of cRNA was measured by using UV spectrophotometry. After cRNA was fragmented, Affymetrix GeneChip® Expression 3' Amplification One-Cycle Target Labeling kit was used. The biotin labeled cRNA molecules were hybridized with GeneChip® Rat Genome 230 2.0 arrays (Affyimetrix), consisting of 31.000 probe sets which analyze the expression level of over 30.000 transcripts and variants from over 28.000 rat genes. Probes were scanned using a GeneChip® Scanner 7G and analysis of the array image was configured using GeneChip® operating software.
Microarray Data Analysis
Normalization of the raw gene expression data was performed using RMA (Robust Multi-Array Average) normalization method in Babelomics gene expression and functional profiling analysis suite (version 4.2) [10]. The normalized intensity values of replicated probes in each array were averaged before performing data analysis. Differentially expressed genes were screened among the four groups; (i) SHAM/ischemic (ii) SHAM/remote zone (iii) MI/ischemic (iv) MI/remote zone by using fold change analysis of Babelomics tool. The fold change threshold was >= 2.0 in defining up or down regulated genes between the groups. Functional characterization of the set of genes obtained from group comparisons was identified using WebGestalt (Web-based Gene Set Analysis Toolkit V2) online tool [11]. The following annotation types were selected for the functional analysis; Gene Ontology (GO) Biological Processes, Molecular Functions, Cellular Components and KEGG (Kyoto Encyclopaedia of Genes and Genomes) Pathways.
RESULTS
Histological Analysis
Histological analysis of infarcts revealed hemorrhagic foci, broadly disorganized interstitial tissue and global disruption of sarcomeric organization including contraction band necrosis (Figure 1 A, B). Infarct histopathology included significant vacuolar regions, reduced cardiomyocyte populations, sarcomeric and cellular disorganization and invasive fibrotic tissue. The region dissected as remote zone (right ventricle), exhibited gross histological characteristics those obtained from the remote zone of shams.
The effect of the experimental protocol apart from the ligation procedure was determined by comparison of expression in sham animals over the study duration. Thus, the effect of experimental MI was superimposed on a dynamic back ground in which sham animals rapidly recovered from the thoracotomy.
Histopathology of the infarct zone obtained after LADC ligation. The upper panels are representative sections of infarct obtained from an animal 30th min after LADC ligation stained with hematoxylin and eosin (A) or trichrome (B). The gross external appearance of this region was unremarkable except for regional discoloration. Histological analysis revealed that tissue integrity was abnormal including regional vacuolation and disruption of myofibrils organization with interstitial hemorrhagic foci and intermittent cellular infiltrates.

Differentially regulated genes in ischemic zones from left ventricles
It was identified that there were 49 differentially regulated transcripts with 2.0 or more fold change after the comparison of the SHAM/ischemic zone and MI/ischemic zone taken from both on left ventricles. While 31 out of 49 transcripts were up-regulated in the MI/ischemic zone, 18 transcripts were altered their expression level in the direction of down-regulation (Supplementary File 1). Total 49 of genes significantly changed in MI/ischemic zone, the up-regulated gene, FBJ osteosarcoma oncogene (Fos), had the highest fold-change, and its fold-change value was 4.5. However, the gene family with sequence similarity 107, member A (Fam107a) with 2.8 fold-changes was the most down-regulated gene in the gene list obtained by performing fold-change screening. To generate both functionally and biologically relevant group of genes, WebGestalt toolkit was used. Differentially regulated genes were grouped into 3 different GO domains (Biological Processes, Molecular Functions and Cellular Components) and their relevant KEGG pathways were identified. GO enrichment analysis of total 49 transcripts showed that the large number of genes was in “binding” (41 genes) (GO: 0005488, p=0.0007), “intracellular” (35 genes) (GO: 0005622, p=0.0377), “response to chemical stimulus” (17 genes) (GO: 0042221, p=3.78e-05) and “response to stress” (13 genes) (GO: 0006950, p=0.0041) ontological categories. Additionally, it was observed a significance in the following GO terms after performing enrichment analysis of significant gene list; “taxis” (5 genes) (GO:0042330, p=5.26e-05), “inflammatory response” (6 genes) (GO:0006954, p=0.0008) and “transcription activator activity” (2 genes) (GO:0016563, p=0.0444) (Table 1). Furthermore, it was found significantly changed 6 KEGG pathways in response to coronary artery-ligation in MI/ischemic zone (Table 2) (p<0.05).
GO enrichment analysis MI ischemic and remote zones in response to LCAL.
| Gene Ontology term | SHAM/ischemic vs. MI/ischemic zone | SHAM/remote vs. MI/remote zone | ||
|---|---|---|---|---|
| p-value | # of Genes | p-value | # of Genes | |
| - Response to chemical stimulus (GO:0042221) | 3.78e-05 | 17 | 0.0021 | 27 |
| -Response to corticosteroid stimulus (GO:0031960) | 0.0030 | 4 | 0.0041 | 6 |
| - Chemotaxis (GO:0006935) | 5.26e-05 | 5 | 0.0038 | 5 |
| - Cell chemotaxis (GO:0060326) | 4.53e-05 | 4 | 0.0016 | 4 |
| - Leukocyte chemotaxis (GO:0030595) | 3.37e-05 | 4 | 0.0012 | 4 |
| - Neutrophil chemotaxis (GO:0030593) | 1.00e-04 | 3 | N/A | not significant |
| - Leukocyte migration (GO:0050900) | 0.0002 | 4 | N/A | not significant |
| - Immune response (GO:0006955) | 0.0004 | 8 | N/A | not significant |
| - Inflammatory response (GO:0006954) | 0.0008 | 6 | N/A | not significant |
| - Chemokine activity (GO:0008009) | 1.71e-07 | 5 | N/A | not significant |
| - Cytokine activity (GO:0005125) | 5.55e-05 | 5 | N/A | not significant |
| - Catecholamine metabolic process (GO:0006584) | 0.0128 | 2 | N/A | not significant |
| - Binding (GO:0005488) | 0.0007 | 41 | 0.0085 | 104 |
| - Protein binding (GO:0005515) | 0.0123 | 32 | 0.0120 | 80 |
| -Cytokine receptor binding (GO:0005126) | 1.00e-04 | 5 | N/A | not significant |
| -Chemokine receptor binding (GO:0042379) | 2.02e-07 | 5 | 0.0005 | 4 |
| - Calcium ion binding (GO:0005509) | 0.0463 | 5 | 0.0083 | 12 |
| - Heparin binding (GO:0008201) | 0.0277 | 2 | N/A | not significant |
| - Intracellular (GO:0005622) | 0.0377 | 35 | 0.0006 | 94 |
| - Actin cytoskeleton (GO:0015629) | 0.0055 | 4 | N/A | not significant |
| - Myosin complex (GO:0016459) | 0.0004 | 3 | N/A | not significant |
| - Response to vitamin (GO:0033273) | not significant | N/A | 0.0012 | 6 |
| - Response to calcium ion (GO:0051592) | not significant | N/A | 0.0052 | 4 |
| - Response to amino acid stimulus (GO:0043200) | not significant | N/A | 0.0006 | 4 |
| - Regulation of anti-apoptosis (GO:0045767) | not significant | N/A | 0.0010 | 4 |
| - Caveola assembly (GO:0070836) | not significant | N/A | 1.00e-04 | 2 |
| - Regulation of cytoskeleton organization (GO:0051493) | not significant | N/A | 0.0002 | 7 |
| -Regulation of microtubule cytoskeleton organization (GO:0070507) | not significant | N/A | 0.0011 | 4 |
| -Actin polymerization or depolymerization (GO:0008154) | not significant | N/A | 0.0033 | 4 |
| - Regulation of endothelial cell proliferation (GO:0001936) | not significant | N/A | 0.0036 | 3 |
| - Lipid binding (GO:0008289) | not significant | N/A | 0.0090 | 9 |
| -Diacylglycerol binding (GO:0019992) | not significant | N/A | 0.0079 | 3 |
| -Fatty acid binding (GO:0005504) | not significant | N/A | 0.0007 | 4 |
| - Mitochondrion (GO:0005739) | not significant | N/A | 0.0113 | 22 |
| - Cytoplasmic vesicle (GO:0031410) | not significant | N/A | 0.0195 | 12 |
| -Perinuclear region of cytoplasm (GO:0048471) | not significant | N/A | 0.0425 | 6 |
| - Cytosol (GO:0005829) | not significant | N/A | 0.0006 | 24 |
| -Ribonucleoprotein complex (GO:0030529) | not significant | N/A | 0.0079 | 9 |
| - Envelope (GO:0031975) | not significant | N/A | 0.0197 | 11 |
| -Cytoplasmic membrane-bounded vesicle (GO:0016023) | not significant | N/A | 0.0219 | 11 |
| - Mitochondrial envelope (GO:0005740) | not significant | N/A | 0.0389 | 8 |
Differentially regulated genes in remote zone on right ventricles
It was found that 143 genes were differentially regulated between the SHAM/remote zone and MI/remote zone groups taken from both on right ventricles (Supplementary File 2). While 32 out of 143 genes were significantly down-regulated, other genes were increased in their expression level 2.0 or more as fold change. Among the differentially expressed genes, Caveolin-1 (Cav1) was the most increased gene with 4.6 fold change. Additionally, Family with sequence similarity 111, member A (Fam111a) and 3-Phosphoinositide dependent protein kinase-1 (Pdpk1) genes were the most two down-regulated genes with 2.9 fold change.
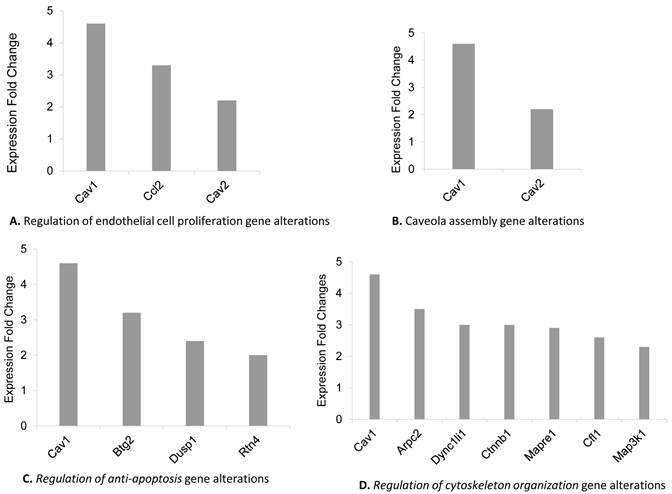
GO functional enrichment analysis of the both up and down-regulated genes in remote zones was revealed the significance of distinct GO terms as shown in Table 1. We found that differentially regulated genes mainly were collected in certain GO terms including “response to chemical stimulus” (27 genes) (GO:0042221, p=0.0021), “response to stress” (25 genes) (GO:0006950, p=0.0059), “response to abiotic stimulus” (15 genes) (GO:0009628, p=2.35e-05), “binding” (104 genes) (GO:0005488, p=0.0085) and “intracellular part” (94 genes) (GO:0044424, p=0.0006) “Caveola assembly” (2 genes) (GO:0070836 p= 1.00e-04), “Regulation of anti-apoptosis” (2 genes) (GO:0045767 p=0.0010), “Regulation of cytoskeleton organization” (7 genes) (GO:0051493 p= 0.0002) “Regulation of endothelial cell proliferation” (3 genes) (GO:0001936 p= 0.0036). Furthermore, GO term enrichment analysis of the gene set including 143 differentially regulated genes showed significance in the following GO terms; “regulation of endothelial cell proliferation” (3 genes) (GO:0001936, p=0.0036), “taxis” (5 genes) (GO:0042330, p=0.0038) “sarcoplasm” (3 genes) (GO:0016528, p=0.0075) and “vesicle” (12 genes) (GO:0031982, p=0.0309). The most remarkable gene alterations found in only remote zone were grouped as “regulation of endothelial cell proliferation”, “Caveola assembly” “Regulation of anti-apoptosis”, and “Regulation of cytoskeleton organization” Go terms showed in figure 2 A, B, C, D respectively. Behind to the analysis of GO term distribution of the significant genes, KEGG pathway analysis identified 11 pathways which were significantly altered by comparing the remote zones of the groups (Table 2) (p<0.05).
KEGG pathways in response to LCAL in MI ischemic and remote zones.
| KEGG Pathway name | Corresponding genes | p-value |
|---|---|---|
| SHAM/ischemic zone and MI/ischemic zone comparison | ||
| NOD-like receptor signaling pathway | Cxcl2, Cxcl1, Ccl2, Ccl7 | 1.65e-05 |
| Chemokine signaling pathway | Cxcl2, Cxcl1, Ccl2, Ccl7, Cxcl10 | 0.0002 |
| Focal adhesion | Col1a1, Pdpk1, Mylpf, Arhgap5 | 0.0033 |
| Leukocyte transendothelial migration | Mylpf, Arhgap5, Cybb | 0.0063 |
| Cytokine-cytokine receptor interaction | Cxcl2, Ccl2, Cxcl10 | 0.0172 |
| Toll-like receptor signaling pathway | Fos, Cxcl10 | 0.0260 |
| SHAM/remote zone and MI/remote zone comparison | ||
| Adherens junction | Actb, Ctnnb1, Csnk2a1 | 0.0315 |
| Tight junction | Prkci, Actb, Ctnnb1, Myl7, Ppp2r1a, Csnk2a1 | 0.0014 |
| Focal adhesion | Col1a1, Actb, Pdpk1, Cav1, Ctnnb1, Myl7, Cav2 | 0.0022 |
| Regulation of actin cytoskeleton | Arpc2, Cfl1, Pfn1, Actb, Myl7 | 0.0432 |
| Viral myocarditis | Actb, Cav1, Eif4g2, RT1-Ba | 0.0027 |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | Cacna2d1, Dsc2, Actb, Ctnnb1 | 0.0039 |
| NOD-like receptor signaling pathway | Cxcl1, Hsp90ab1, Ccl2 | 0.0103 |
| PPAR signaling pathway | Fabp4, Pdpk1, Dbi | 0.0315 |
| Ubiquitin mediated proteolysis | Ube2g1, Ube2s, Map3k1, Nedd4 | 0.0309 |
| Spliceosome | Nhp2l1, Snrpf, Lsm7, Smndc1 | 0.0258 |
| Ribosome | Rps10, Rps24, Rpl34 | 0.0354 |
SHAM/remote zone and MI/remote zone comparison of altered genes in Gene Ontology terms.
Common differentially regulated genes in ischemic zones from left ventricles and remote zone from right ventricles
There were only 9 altered transcripts which were identified as common genes in remote and ischemic zones. It was found that the genes chemokine (C-C motif) ligand 2 (Ccl2), chemokine (C-X-C motif) ligand 10 (Cxcl10) and chemokine (C-X-C motif) ligand 1 (melanoma growth stimulating activity, alpha) (Cxcl1) were up-regulated in both remote and ischemic zones of the rats. CD93 molecule (Cd93), Pdpk1 and calcium channel, voltage-dependent, alpha2/delta subunit 1 (Cacna2d1) were up-regulated in ischemic zone; however, those were identified as down-regulated in remote zone. Additionally, the genes collagen, type I, alpha 1 (Col1a1), DDB1 and CUL4 associated factor 6 (Dcaf6) and prefoldin subunit 6 (Pfdn6) were down-regulated in ischemic zones of the coronary artery-ligated rats while those were up-regulated in the remote zone.
DISCUSSION
Acute myocardial infarction is the interruption of blood supply to a part of the heart, causing heart cells to die. This is most commonly due to occlusion of a coronary artery following the rupture of a vulnerable atherosclerotic plaque, which is an unstable collection of lipids and white blood cells mostly macrophages in the wall of an artery. The resulting ischemia and oxygen shortage, if left untreated for a sufficient period of time, can cause damage or death of heart muscle tissue. Despite modern reperfusion therapies, acute myocardial infarction remains associated with the development of heart failure in a significant proportion of patients [12, 13]. Many therapeutic approaches designed to limit injury after infarct are based on empirical observation of efficacious clinical outcomes associated with myocardial reperfusion or reduced cardiac work. These include: 1. Pharmacological manipulation e.g. angiotensin-converting-enzyme (ACE) inhibitors, β-adrenergic blockers (β-blockers) and statins, 2. Coronary artery bypasses graft surgery and 3. Interventional cardiology methods for example stents and angioplasty. Recently, it has been suggested that maladaptive changes in cardiac contractility are associated with activation of a fetal gene program by pathologic stimuli [14- 18]. While the data supporting this idea are limited, validation of this hypothesis could provide novel targets for therapeutic intervention after MI either at the level of transcription or via the inductive signal transduction pathways triggering these pathways [16]. In this study we hypothesized that there may be global transcriptional alterations in right ventricle which is accepted as remote zone as an early response against ischemia following myocardial infarction at left ventricle. These molecular alterations give us new insights for the novel therapeutic targets against AMI or to understand how collaterals improved without any lethal damage in the heart tissues.
MI response attributes to regional effects of ischemia on several physiological systems across diverse cardiac components including the contractile apparatus, the extracellular matrix (ECM) and the vasculature [16, 19]. In many studies, increased levels of different markers of inflammation have been associated with AMI as an early response within 24 h. In our study it has been shown that immune response was one of the most remarkable results as an immediate response (30min) in after ligation of the left anterior descending coronary artery (LADCA) in rats. At this point we should emphasize that the measured molecular responses to AMI in the infarct and remote location were performed at 30 minutes only. Measurements either earlier of later would be helpful in future experiments to assess the progression and change in molecular responses to AMI.
This immune response has been validated with other significantly changed conditions such as inflammatory response, chemokine activity, cytokine activity, cytokine receptor binding, neutrophil chemotaxis, and leukocyte chemotaxis and leukocyte migration. Furthermore, expression of chemokine ligands (Cxcl1, Ccl2, Cxcl2, Ccl7, and Cxcl10) increased in the infarct zone. It is known that these ligands recruit leucocytes (such as monocytes), memory T cells, and dendritic cells to sites of tissue injury, infection, and inflammation [20]. Hence, it is expected to an increase in chemotaxis of immune cells towards infarct zone as following increase level of ligands. Moreover, Ca++ ion binding related genes also deregulated in transcriptional level in the ischemic zone in MI rat. While level of Cacna2d1 increased, Myosin light chain 3, skeletal muscle isoform (Myl1), Myosin Light Chain, Phosphorylatable, Fast Skeletal Muscle (Mylpf) and Troponin C type 2 (Tnnc2) transcripts decreased under the effect of ischemia. This molecular signature shows us the ischemia possibly causes loss of muscle construction due to alterations in Ca current density as a sudden effect. The alterations in the expression of Myl1 and Mylpf genes emphasized that change in Ca level effects on cytoskeleton organization of myocytes in ischemic area. Indeed, regulation of actin cytoskeleton, myosin complex and contractile fiber related genes were significantly changed in ischemic zone.
Although dynamic changes in distinct remote and ischemic zones were studied in AMI rat model, there was no data available for the alterations in the area out of left ventricle in which MI produced by ligation. As the first time in literature we use of right ventricle as remote zone in the present study. Histological analysis proved no ischemia in the right ventricle. Interestingly, there were several significant alterations in the level of transcript of genes which can be grouped in immune response, cytoskeleton organization, angiogenesis, and mitochondrial metabolism. Although immune response, cytoskeleton organization and calcium metabolism related genes were changed in a similar manner in both remote and ischemic zones, anti-apoptosis, lipid metabolism, endothelial cell proliferation and Caveolae- related genes were deregulated only in remote zone as early response to AMI. Lipid rafts represent a subcompartment of the plasma membrane that coordinate and regulate varieties of signaling processes while caveolins are the integral membrane protein of the lipid raft. It is demonstrated that lipid raft play a pivotal role in the generation of survival signal in preconditioning or adapted heart and disintegration of lipid raft completely abolish cardioprotection [19]. Caveolae, lipid-rich microdomains of the sarcolemma, localize and enrich cardiac-protective signaling. Cav1 is the main coat protein of caveolae and is expressed by different vascular cells [22-24]. Caveolae and Cav1 have emerged as novel targets in the control of various important cellular processes involved in the maintenance of cardiovascular homeostasis such as protein trafficking, lipid metabolism and signal transduction [25]. Cardiac-specific overexpression of Caveolin 3 (Cav3) induces endogenous cardiac protection by mimicking ischemic/preconditioning [20]. In the present study, Cav1 and Caveolin 2 (Cav2) genes were significantly up regulated in remote zone. Thus, we suggested that remote zone might sense ischemia with an unknown mechanism and altered lipid raft configuration to provide signals for cardioprotection and/or endothelial cell proliferation. The other very interesting result was alterations on the mitochondrion, mitochondrial envelope and regulation of anti-apoptosis gene in remote zone. Mitochondria are the main organelle in cell for energy metabolism and the control of intrinsic way of apoptosis. So, it is acceptable to increase ATP hydrolysis and anti-apoptotic genes in remote zone as we found in our analysis. Because cells in remote zones possibly intended to survive and proliferate toward ischemic zone as a very early response. Moreover, analysis our data in KEGG pathways showed us tight junction, adherent junction, focal adhesion genes were also deregulated. These deregulations were highly similar with viral myocarditis and arrhythmogenic right ventricular cardiomyopathy [26-29]. Cytoskeleton, mitochondrial and lymphocytic changes marked viral myocarditis and desmosome, cytoskeleton, inflammation, calcium transport and apoptosis changes indicates the appearance of similar phenotypic alterations with arrhythmogenic right ventricular cardiomyopathy and dilated cardiomyopathy in the remote zone [19, 20].
As a conclusion molecular findings supposed that signals through the infarct zone is able to force remote zone to survive by the way of endothelial proliferation, angiogenesis, mitochondrial changes, apoptotic regulation. Validation of this hypothesis could provide novel targets for therapeutic intervention after MI.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Troidl C, Möllmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsässer A. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485-96
2. Hong D, Zeng X, Xu W, Ma J, Tong Y, Chen Y. Altered profiles of gene expression in curcumin-treated rats with experimentally induced myocardial infarction. Pharmacol Res. 2010;61:142-8
3. Zhao W, Zhao T, Huang V, Chen Y, Ahokas RA, Sun Y. Platelet-derived growth factor involvement in myocardial remodeling following infarction. J Mol Cell Cardiol. 2011;51:830-8
4. Dębiński M, Buszman PP, Milewski K, Wojakowski W, Jackiewicz W, Pająk J, Szurlej D, Fryc-Stanek J, Wiernek S, Jelonek M, Spurlock ME, Martin J, Bochenek A, Buszman PE. Intracoronary adiponectin at reperfusion reduces infarct size in a porcine myocardial infarction model. Int J Mol Med. 2011;27:775-81
5. Katayama Y, Takaji K, Shao ZQ, Matsukawa M, Kunitomo R, Hagiwara S, Moriyama S, Kawasuji M. The value of angiogenic therapy with intramyocardial administration of basic fibroblast growth factor to treat severe coronary artery disease. Ann Thorac Cardiovasc Surg. 2010;16:174-80
6. Iwasaki H, Kawamoto A, Tjwa M, Horii M, Hayashi S, Oyamada A, Matsumoto T, Suehiro S, Carmeliet P, Asahara T. PlGF Repairs Myocardial Ischemia through Mechanisms of Angiogenesis, Cardioprotection and Recruitment of Myo-Angiogenic Competent Marrow Progenitors. PLoS One. 2011;6:24872
7. Baxter GF, Goma FM, Yellon DM. Involvement of protein kinase C in the delayed cytoprotection following sublethal ischaemia in rabbit myocardium. Br J Pharmacol. 1995;115:222-4
8. Szabó G, Veres G, Radovits T, Gero D, Módis K, Miesel-Gröschel C, Horkay F, Karck M, Szabó C. Cardioprotective effects of hydrogen sulfide. Nitric Oxide. 2011;25:201-10
9. Calvert JW, Elston M, Nicholson CK, Gundewar S, Jha S, Elrod JW, Ramachandran A, Lefer DJ. Genetic and pharmacologic hydrogen sulfide therapy attenuatesischemia-induced heart failure in mice. Circulation. 2010;122:11-9
10. Medina I, Carbonell J, Pulido L, Madeira SC, Goetz S, Conesa A, Tárraga J, Pascual-Montano A, Nogales-Cadenas R, Santoyo J, García F, Marbà M, Montaner D, Dopazo J. Babelomics: An integrative platform for the analysis of transcriptomics, proteomics and genomic data with advanced functional profiling. Nucleic Acids Res. 2010;38:210-3
11. Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:741-8
12. Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, Caplin JL, Rigby AS, Goode K. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. 2008;29:859-70
13. Devaux Y, Azuaje F, Vausort M, Yvorra C, Wagner DR. Integrated protein network and microarray analysis to identify potential biomarkers after myocardial infarction. Funct Integr Genomics. 2010;10:329-37
14. Lyn D, Liu X, Bennett NA, Emmett NL. Gene expression profile in mouse myocardium after ischemia. Physiol Genomics. 2000;2:93-100
15. Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000;86:939-45
16. Chugh SS, Whitesel S, Turner M, Roberts CT Jr, Nagalla SR. Genetic basis for chamber-specific ventricular phenotypes in the rat infarct model. Cardiovasc Res. 2003;57:477-85
17. Chien KR, Olson EN. Converging pathways and principles in heart development and disease: CV@CSH. Cell. 2002;110:153-62
18. LaFramboise WA, Bombach KL, Dhir RJ, Muha N, Cullen RF, Pogozelski AR, Turk D, George JD, Guthrie RD, Magovern JA. Molecular dynamics of the compensatory response to myocardial infarct. J Mol Cell Cardiol. 2005;38:103-17
19. Colucci WS. Molecular and cellular mechanisms of myocardial failure. Am J Cardiol. 1997;80:15-25
20. Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010-20
21. Das M, Das S, Lekli I, Das DK. Caveolin induces cardioprotection through epigenetic regulation. J Cell Mol Med. 2011 [Epub ahead of print]
22. Tsutsumi YM, Horikawa YT, Jennings MM, Kidd MW, Niesman IR, Yokoyama U, Head BP, Hagiwara Y, Ishikawa Y, Miyanohara A, Patel PM, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation. 2008;118:1979-88
23. Rodriguez-Feo JA, Hellings WE, Moll FL, De Vries JP, van Middelaar BJ, Algra A, Sluijter J, Velema E, van den Broek T, Sessa WC, De Kleijn DP, Pasterkamp G. Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease. PLoS One. 2008;3:2612
24. Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408-17
25. Frank PG, Hassan GS, Rodriguez-Feo JA, Lisanti MP. Caveolae and caveolin-1:novel potential targets for the treatment of cardiovascular disease. Curr Pharm Des. 2007;13:1761-9
26. Oxford EM, Everitt M, Coombs W, Fox PR, Kraus M, Gelzer AR, Saffitz J, Taffet SM, Moïse NS, Delmar M. Molecular composition of the intercalated disc in a spontaneous canine animal model of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2007;4:1196-205
27. McCauley MD, Wehrens XH. Animal models of arrhythmogenic cardiomyopathy. Dis Model Mech. 2009;2:563-70
28. Nikolova-Krstevski V, Leimena C, Xiao XH, Kesteven S, Tan JC, Yeo LS, Yu ZY, Zhang Q, Carlton A, Head S, Shanahan C, Feneley MP, Fatkin D. Nesprin-1 and actin contribute to nuclear and cytoskeletal defects in lamin A/C-deficient cardiomyopathy. J Mol Cell Cardiol. 2011;50:479-86
29. Frazier AH, Ramirez-Correa GA, Murphy AM. Molecular mechanisms of sarcomere dysfunction in dilated and hypertrophic cardiomyopathy. Prog Pediatr Cardiol. 2011;31:29-33
Author contact
![]() Corresponding author: Cenk Erdal, Department of Cardiovascular Surgery, Dokuz Eylül University, Faculty of Medicine, 35340 İnciraltı, İzmir, TURKEY. Phone: + 90 505 394 3627 e-mail: cenk.erdaledu.tr
Corresponding author: Cenk Erdal, Department of Cardiovascular Surgery, Dokuz Eylül University, Faculty of Medicine, 35340 İnciraltı, İzmir, TURKEY. Phone: + 90 505 394 3627 e-mail: cenk.erdaledu.tr

 Global reach, higher impact
Global reach, higher impact