3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(8):704-708. doi:10.7150/ijms.8.704 This issue Cite
Research Paper
The Effect of Irrigation Temperature on Bone Healing
Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Istanbul University, Istanbul, Turkey.
Received 2011-8-19; Accepted 2011-10-18; Published 2011-10-22
Abstract
Objective: Osteotomies, performed by rotational instruments, can cause temperature rise on the bone and elevated temperature can disrupt the bone healing. When the osteotomies are performed for the insertion of miniscrews, the bone healing disruption may cause stability loosening or failures. Saline irrigation is mostly used for the prevention of the heat generation during osteotomy.
Purpose: The purpose of this study was to evaluate the effect of the saline irrigation temperature on bone healing.
Material and Method: Standardized drilling and miniscrew placement was performed in the tibias of 18 Sprague Dawley rats with rotating bur uncooled, cooled with 25°C and 4°C saline irrigations. After the 21 days, the difference in healing was observed between the uncooled and cooled groups.
Results: Although there was no statistically significant difference between the group irrigated with 25°C and 4°C saline for newly bone formation, osteoblasts were seen more active and bone marrow was more dynamic in group 4°C than group 25°C. There is no disadvantage to use 25°C, but it may be better to use 4°C for rapid healing.
Keywords: bone healing, saline irrigation temperature
Introduction
Dental implantation and fixation of screws for any reason are performed by drilling the bone and the success of these operations depend on many factors. Through these factors, thermal injuries occurring due to temperature raise during drilling, may be the most influent one (1,2). The threshold level for thermal injuries on the bone is the 47°C for a minute and the temperature can raise that level easily during drilling by rotational burs (1,3). As a consequence of thermal injury, bone is not only resorbed but also replaced with fat cells (4). As a result, the mechanical structure of the bone is weaken (5). To prevent the bone from the temperature raise during drilling, various irrigation systems are used and mostly, sterile saline solutions are the material of choice (6,7). Although, to our knowledge, there is not a scientific data in the literature, there is a belief among the surgeons that cooled saline irrigation is more effective than the uncooled saline irrigation to protect the bone from the thermal injuries.
The purpose of the present study was to investigate the effect of the irrigation temperature on the bone healing. For this aim, standardized drilling and miniscrew placement was performed in the tibias of 18 Sprague Dawley rats with rotating bur uncooled, cooled with 25°C and 4°C saline irrigation. The bone healing was evaluated between the uncooled and cooled groups.
Material and Method
Experiments were designed to determine the effect of irrigation temperature on the bone after osteotomy and miniscrew placement. For this aim 3 months old weighing approximately 350 to 450 g 18 Sprague-Dawley rats were maintained at 22 ±0.5 °C on a 12-h light/12-h dark cycle with free access to water and standartized food in pathogen free separate cages. All of the experiments were performed at the Istanbul University, Institutes of Experimental Science Laboratories (Istanbul, Turkey). Maintenance and all experimental procedures performed were fully in accordance with principles established by the existing governmental acts and approved by the University Institutional Animal Welfare Committee.
Animals were divided randomly in to 3 groups of 6 animals and 3 experiments were performed. Anesthesia was induced with ketamine HCl (100 mg/kg i.p., Ketalar ®, Parke Davis) by the injection through the peritonium of the rats and maintained with xylozin HCl (23.32 mg/ml i.p., Rompun®, Bayer). After the induction of anesthesia, operation sites were shaved and the rats were placed and stabilized on the operation table. All of the animals were operated by the same surgeon. Skin incision was performed parallel to the long axis of the right femurs and the bone was exposed by the dissection of hamstring and quadriceps femoris muscles.
Three experimental procedures were performed. Three drilling and miniscrew placement were performed on each animal. Totally 54 drilling and miniscrew placement was done. The drill speed was set to be 20,000 rpm, which is similar to that used during osteotomies by rotational systems and saline irrigation speed was standartized by using saline-dispencer. All of the holes were drilled with standard burs manufactured for 2mm miniscrews and standart 2mm wide 5mm length titanium miniscrews (Bone Screw Kit, Biohorisons®, USA) were inserted.
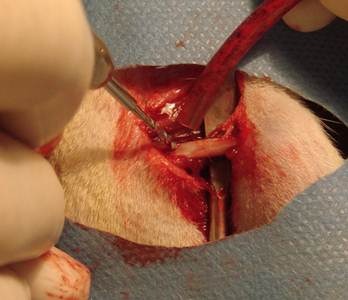
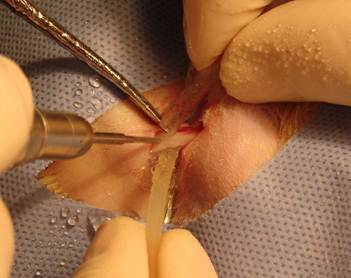
In the group one, holes were drilled without irrigation (Figure 1). In the group two, holes were drilled with 25°C (±2°C) saline irrigation and in the group three, holes were drilled with 4°C (±2°C) saline irrigation (Figure 2).
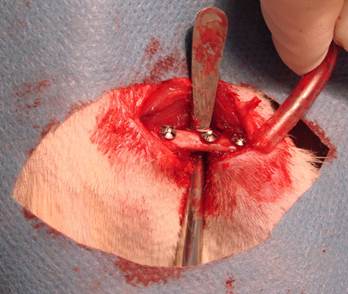
After the drilling and the miniscrew placement (Figure 3), muscle and fascia layers were closed with simple resorbable 4.0 sutures (Vicryl 4-0, Ethicon) and finally the skin incisions were closed with continious 3.0 sutures (Silk 3.0, Dogsan). Postoperative antibiotics (gentamicin, 0.5 mg/kg) and analgesics (xylocaine 0,5 mg/kg) were given subcutaneously. The positions of the miniscrews were controlled by cone beam CT scanning. During post-operative period, all of the animals were controlled day by day and none of them were lost.
Drilling without irrigation
Drilling with 25°C and +4°C saline solution
Placement of miniscrews
At the end of three weeks, animals were killed and right femurs of them were harvested. Then, the specimens were sent to the pathology laboratory (Istanbul University, Institute of Oncology, Department of Pathology, Istanbul, TURKEY) for the histopathological evaluation. The cutting line was started parallel to the transverse axis of femurs at about 4 mm apart from femoral heads, where the first hole preparation was made. The specimens were fixed in 10% formalin for one week and decalcified in 10% formic acid solution (Merck, Darmstadt, Germany) for 25 days. The decalcified specimens were embedded in paraffin and cut into 3 µm thick sections on charged slides using a microtome (Leica Microsystemic RM 2125, Germany), and routine hematoxylin and eosin (H&E) staining was performed. The sections were examined with a light microscope (Olympus BX60 microscope) attached to a digital camera (Olympus E-330) which connected to a computer. A histomorphological review was performed by a single blinded oral pathologist to evaluate the presence of infection, necrosis, fibrosis and new bone formation. All screw hole surroundings were evaluated by histopathologically with light microscope under 20, 40, 100 and 200x magnifications.
The score was made up of 0-5% as (-), 5-30% as (+), 30-60% as (++) and 60% and over as (+++) to evaluation of new bone formation and fibrosis according to their surface covering at 40x magnification. The scores for infection and necrosis were determined by occurring in a standardized area at 20x magnification. The infection was determined if inflammatory cells were observed in the healing area the (+) score and if there were no inflammatory cells the (-) score were given. For necrosis the (-) score was given, if there was no necrosis whereas the (+) score was given if there was.
The statistical differences between the control and test groups were compared by ki-square and Fisher tests.
Results
In this research, all of the statistical evaluations were performed by using the NCSS software (NCSS Inc., 2007, USA). Ki-square and Fisher tests were performed for the evaluation of the definitive statistical methods and also qualitative datas. Probabilities of less than 0.05 were considered significant (p<0,05).
The distribution difference of the new bone formation between 4°C, 25°C and control groups were observed as statistically significant (p=0,031)(Table1). The new bone formation of the control group was evaluated significantly lower than the 4°C and 25°C groups (p=0,020, p=0,049) , however, there was no statistically significant difference evaluated between the 4°C and 25°C groups statistically(p=0,637)(Table 2).
The distribution difference of the new bone formation.
| New Bone Formation | Group 4°C | Group 25°C | Control Group | ||||
|---|---|---|---|---|---|---|---|
| (-) | 0 | 0,00% | 0 | 0,00% | 3 | 25,00% | |
| (+) | 3 | 25,00% | 4 | 33,30% | 7 | 58,30% | |
| (++) | 5 | 41,70% | 6 | 50,00% | 2 | 16,70% | χ²:13,86 |
| (+++) | 4 | 33,30% | 2 | 16,70% | 0 | 0,00% | p=0,031 |
Statistical difference of the new bone formation between groups.
| New Bone Formation | |
|---|---|
| Group 4°C / Group 25°C | 0,637 |
| Group 4°C / Control Group | 0,020 |
| Group 25°C / Control Group | 0,049 |
Statistically significant difference was observed between 4°C, 25°C and control groups regarding the distribution of the infection presence (p=0,0001)(Table3). The presence of the infection in the control group 9 (%75), was observed as significantly higher than the 4°C and 25°C groups (p=0,0001) , but no statistically significant difference was seen between the 4°C and 25°C groups (p=1)(Table 4).
The distribution difference of the infection presence.
| Infection | Group 4°C | Group 25°C | Control Group | ||||
|---|---|---|---|---|---|---|---|
| (-) | 12 | 100,00% | 12 | 100,00% | 3 | 25,00% | χ²:24,00 |
| (+) | 0 | 0,00% | 0 | 0,00% | 9 | 75,00% | p=0,0001 |
Statistical difference of the infection presence between groups.
| Infection | |
|---|---|
| Group 4°C / Group 25°C | |
| Group 4°C / Control Group | 0,0001 |
| Group 25°C / Control Group | 0,0001 |
The distribution of the bone necrosis existence between 4°C, 25°C and control groups were observed significantly different (p=0,0001) (Table 5). The existence of the bone necrosis in the control 11 (%91,7), group was evaluated significantly higher than the 4°C and 25°C groups (p=0,0001), but, no statistically difference was observed between the 4°C and 25°C groups (p=1) (Table 6).
The distribution of the bone necrosis existence.
| Bone Necrosis | Group 4°C | Group 25°C | Control Group | ||||
|---|---|---|---|---|---|---|---|
| (-) | 12 | 100,00% | 12 | 100,00% | 1 | 8,30% | χ²:31,68 |
| (+) | 0 | 0,00% | 0 | 0,00% | 11 | 91,70% | p=0,0001 |
Statistical difference of the bone necrosis existence between groups.
| Bone Necrosis | |
|---|---|
| Group 4°C / Group 25°C | |
| Group 4°C / Control Group | 0,0001 |
| Group 25°C / Control Group | 0,0001 |
No statistically significant difference was observed between 4°C, 25°C and control groups regarding the distribution of the fibrosis existence (p=0.355)(Table7). The fibrosis distribution of the control group was not significantly different than the 4°C and 25°C groups (p=0,334, p=0,164), and also, no statistically significant difference was observed between the 4°C and 25°C groups (p=0,232) (Table 8).
The distribution of the fibrosis existence.
| Fibrosis | Group 4°C | Group 25°C | Control Group | ||||
|---|---|---|---|---|---|---|---|
| (-) | 2 | 16,70% | 5 | 41,70% | 5 | 41,70% | |
| (+) | 3 | 25,00% | 0 | 0,00% | 3 | 25,00% | |
| (++) | 5 | 41,70% | 5 | 41,70% | 4 | 33,30% | χ²:6,64 |
| (+++) | 2 | 16,70% | 2 | 16,70% | 0 | 0,00% | p=0,355 |
Statistical difference of the fibrosis existence betweeem groups.
| Fibrosis | |
|---|---|
| Group 4°C / Group 25°C | 0,232 |
| Group 4°C / Control Group | 0,334 |
| Group 25°C / Control Group | 0,164 |
Discussion
The temperature rise on the bone during osteotomies performed by rotational systems is affected by various factors (8-10). Some of these factors are bone density, and the location of the osteotomy, in particular the amount of cortical versus cancellous bone, which may be influential. Other important factors pertain to the drilling, including the speed at which the drill rotates, the sharpness of the margins on the drill, the thickness of the drill, and the force with which the drills applied to the bone (11). Regardless of the reason for, this temperature rise can cause damage or impaired healing on the bone (1,3). As previously mentioned, the accepted threshold level that is required for the thermal injury on the bone is 47°C for a period of 1 minute (1,3).
For the protection of the bone from the thermal damage during osteotomy, saline solution is routinely applied to the drill and the osteotomy site in surgical practice. And also, most of the surgeons prefer cool saline solutions and they believe that it is more effective than the normal solutions for the reduction of the temperature. In the literature, there are some studies indicating the effect of the saline application on temperature rise (11) , on the contrary , others indicate that application of saline solution to the rotational system to bone interface during osteotomy do not reduce the temperature during the osteotomy to any significant degree (12,13). In the authors' knowledge, this is the first study focuses on the effect of the irrigation temperature to reduce thermal rise. At the same time, it is evaluated in this study that application of saline solution is effective or not for the reduction of the thermal damage.
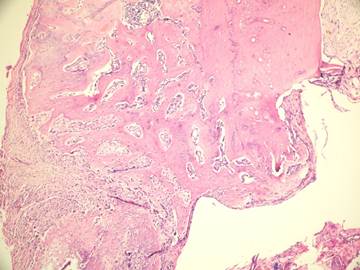
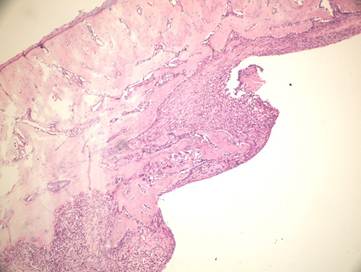
In the present study we evaluated the specimens in terms of new bone formation, presence of infection, necrosis and fibrosis Our study demonstrated significant difference regarding the necrosis values between control group (without irrigation) and the group irrigated with 25°C and 4°C saline (Figures 4-6). Necrosis was not observed in second and third groups. However copious saline irrigation was useful for cleaning the operation area from any remnants of the drill. It is thought that this can be an advantage for wound healing.
Although there was no statistically significant difference between the group irrigated with 25°C and 4°C saline for new bone formation, latter group had numerous osteoblasts and more prominent osteoblastic rim around the trabeculae of new bone (Figures 4,5). These results indicate that there is no disadvantage to use 25°C saline irrigation, but it may be better to use 4°C saline irrigation for rapid healing.
Newly formed bone in group +4°C (H&E x100)
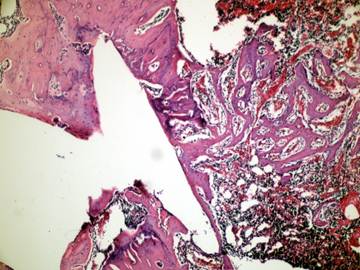
Fibrosis and new bone formation in group +25°C (H&E x100)
Inflammatory cells and new bone formation in control group (without irrigation, H&E
In this study, it is verified that the use of saline irrigation is not only useful to reduce temperature rise on the bone during osteotomy, but also effective for cleaning the osteotomy site from any bony remnants.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Eriksson A, Albrektsson T, Grace B, McQueen O. Thermal injury to bone. A vital microscopic description of heat effects. Int J Oral Surg. 1982;11:115-21
2. Eriksson R, Adell R. Temperatures during drilling for the placement of implants using the osteointegration technique. J Oral Maxillofac Surg. 1986;36:968-73
3. Eriksson R. Heat-induced bone tissue injury. An in vivo investigation of heat tolerance of bone tissue and temperature rise in the drilling of cortical bone. Gothenburg, Sweden: PhD Dissertation, University of Gothenburg, defended February, 21. 1984:1-64
4. Eriksson AR, Albrektsson T. Temperature threshold levels for heatinduced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50(1):101-107
5. Matthews LS, Hirsch C. Temperatures measured in human cortical bone when drilling. J Bone Joint Surg. 1972;54A:297-308
6. Watanabe F, Tawada Y, Komatsu S, Hata Y. Heat distribution in bone during preparation of implant sites: heat analysis by real-time thermography. Int J Oral Maxillofac Implants. 1992;7(2):212-9
7. Kerawala CJ, Martin IC, Allan W, Williams ED. The effects of operator technique and bur design on temperature during osseous preparation for osteosynthesis self-taping screws. Oral Surg Med Oral Pathol Oral Radiol Endod. 1999;88:145-50
8. Brisman DL. The effect of speed, pressure, and time on bone temperature during the drilling of implant sites. Int J Oral Maxillofac Implants. 1996;11:35-7
9. Cordioli G, Majzoub Z. Heat generated during implant site preparation: an in vitro study. Int J Oral Maxillofac Implants. 1997;12:186-93
10. Hobkirk J, Rusiniak K. Investigation of variable factors of drilling bone. J Oral Surg. 1977;35:968-73
11. Hall PB. et al. Thermal Properties of First Metatarsal Osteotomies. J Foot Ankle Surg. 2009;48(4):432-8
12. Larsen ST, Ryd L. Temperature elevation during knee arthroplasty. Acta Orthop Scand. 1989;60(4):439-442
13. Toksvig-Larsen S, Ryd L, Lindstrand A. On the problem of heat generation in bone cutting. Studies on the effects on liquid cooling. J Bone Joint Surg Br. 1991;73(1):13-15
Author contact
![]() Corresponding author: Dr. Erol Cansız, erolcacom
Corresponding author: Dr. Erol Cansız, erolcacom

 Global reach, higher impact
Global reach, higher impact