Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(7):615-622. doi:10.7150/ijms.8.615 This issue Cite
Research Paper
A Double Blind, Randomized, Placebo Controlled Clinical Study Evaluates the Early Efficacy of Aflapin® in Subjects with Osteoarthritis of Knee
1. Department of Orthopedics, Alluri Sitarama Raju Academy of Medical Sciences (ASRAM), National Highway 5, Eluru, 534 002, India
2. Department of Internal Medicine, Alluri Sitarama Raju Academy of Medical Sciences (ASRAM), National High way 5, Eluru, 534 002 India
3. Department of Medicine, Division of Rheumatology, Allergy and Immunology, School of Medicine, U C Davis and VA Medical Center Sacramento, Hospital Way, Mather, California 95655, USA
Received 2011-6-25; Accepted 2011-9-19; Published 2011-10-12
Abstract
Aflapin® is a novel synergistic composition derived from Boswellia serrata gum resin (Indian Patent Application No. 2229/CHE/2008). Aflapin is more efficacious as an anti-inflammatory agent compared to the existing Boswellia products, 5-Loxin® and traditional 65% Boswellia extract. A 30-day, double-blind, randomized, placebo-controlled study was conducted to validate the efficacy of Aflapin® in the management of clinical symptoms of osteoarthritis (OA) of the knee (Clinical trial registration number: ISRCTN69643551). Sixty eligible OA subjects selected through screening were included in the study. The subjects received either 100 mg (n=30) of Aflapin® or placebo (n=30) daily for 30 days. Each subject was evaluated for pain and physical functions by using the standard tools (visual analog scale, Lequesne's Functional Index, and Western Ontario and McMaster Universities Osteoarthritis Index) at the baseline (day 0), and at days 5, 15 and 30. A series of biochemical tests in serum, urine and hematological parameters established the safety of Aflapin. The observations suggest that Aflapin conferred clinically and statistically significant improvements in pain scores and physical function scores in OA subjects. Aflapin provided significant improvements in pain score and functional ability in as early as 5 days of treatment. In conclusion, our observations suggest that Aflapin is a safe, fast acting and effective alternative intervention in the management of OA.
Keywords: Aflapin, Clinical study, Boswellia serrata, Osteoarthritis, Visual Analog Scale
Introduction
Osteoarthritis (OA) is a degenerative joint disorder of articular cartilage and is the most common type of arthritis in elderly persons. In OA, breakdown of cartilage and synovial proliferation result in pain and stiffness of joints. [1-3]. It has been estimated that OA affects more than 27 million people in the United States alone and is the leading cause of physical disability and impaired quality of life in elderly worldwide [4]. Unfortunately, till today there is no proper therapeutic intervention available to treat OA. Currently, acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) including cyclo-oxygenase II inhibitors are used for relief of pain and stiffness [5,6]. Although, these pharmaceutical agents reduce both pain and improve physical functions temporarily without healing the cartilage and subchondral damage, long term usage of NSAIDs is associated with enhanced risk for renal insufficiency, gastrointestinal bleeding, hypertension and congestive heart failure [6-8]. Because of the high incidence of adverse events associated with NSAID therapy, effective and safer alternative treatments for the management of OA pain are highly desirable.
In recent years, the gum resin extracted from the ancient herb, Boswellia serrata has gained considerable attention as a potent anti-inflammatory, anti-arthritic and analgesic agent [9,10]. 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA) is the most active compound of Boswellia extract and is a potent inhibitor of 5-lipoxygenase (5-LOX), a key enzyme in the biosynthesis of leukotrienes from arachidonic acid in the cellular inflammatory cascade [11,12]. A number of independent clinical studies support the anti-inflammatory and anti-arthritic properties of Boswellia extracts [13-16].
Aflapin® is a novel synergistic composition derived from Boswellia serrata gum resin (PCT/IN2009/000505) [17-19]. Interestingly, the oral bioavailability of AKBA from Aflapin was found to be significantly higher in comparison with that of commercially available Boswellia extracts [17]. Aflapin exhibited enhanced 5-lipoxygenase inhibition in enzyme based in vitro assay and Matrix Metalloproteinase 3 (MMP3) inhibition in pro-inflammatory cytokine induced human primary chondrocytes. In a comparative analysis, various in vitro and in vivo studies have established that in comparison with regular Boswellia extracts Aflapin possesses more powerful anti-inflammatory efficacy and exhibits better recovery of glycosaminoglycans (GAG) in pro-inflammatory cytokine induced human chondrocytes. [17]. Furthermore, safety studies conducted according to Organization for Economic Co-operation and Development (OECD) guidelines manifested the overall safety of Aflapin in animal models [18].
In a 90-day placebo controlled clinical study the anti-arthritic efficacy of Aflapin was evaluated in OA subjects. Aflapin demonstrated a significant reduction in pain and improvement in the quality of life in OA subjects [19]. Supplementation of 100 mg Aflapin/day conferred significant improvements in pain scores and physical function. These observations led us to substantiate the anti-OA efficacy of Aflapin in a second independent clinical study. We conducted an independent double blind placebo controlled trial in a different set of subjects with OA. This study design was intended to evaluate, (i) the anti-OA efficacy of Aflapin and (ii) to assess whether Aflapin supplementation can provide fast relief from clinical symptoms of OA. The present communication describes the anti-OA efficacy of Aflapin, which substantiates the earlier observation; and demonstrates that Aflapin provides significant pain relief in subjects with OA in as early as 5 days of treatment.
Materials and Methods
Study material
Aflapin is a novel synergistic composition containing B. serrata extract selectively enriched with AKBA and B. serrata non-volatile oil. The non-volatile oil was prepared by selective removal of Boswellic acids followed by removing volatiles under high vacuum (PCT application # PCT/IN2009/000505). This composition was standardized to contain at least 20% AKBA.
Research design
This randomized, double-blind, placebo controlled trial was conducted during August 2009 to December 2009. The study protocol was approved by the Institutional Review Board (IRB) of Alluri Sitarama Raju Academy of Medical Sciences (ASRAM), Eluru, Andhra Pradesh, India (Clinical Trial Registration No. ISRCTN69643551).
Subjects
One hundred and fifty two patients of either gender were selected for screening. They were between 40 and 80 years of age, and had been suffering from unilateral or bilateral OA of the knee according to the criteria of the American College of Rheumatology [20] for more than 3 months. After the use of usual medications had ceased for 7 days, the visual analog scale (VAS) score that assessed pain during the most painful knee movement had to be more than 40, and Lequesne's functional index [21] had to be over 7 points. Participants had to be able to walk and give both verbal and written information regarding the study. Signed informed consent was obtained prior to entry. Exclusion criteria included an underlying inflammatory arthropathy, hyperuricemia, expectation of surgery in the near future, recent injury in the area affected by OA of the knee, intra-articular corticosteroid injections within the last 3 months, hypersensitivity to NSAIDs, abnormal liver or kidney function tests, major abnormal finding on complete blood count, history of coagulopathies, history of peptic ulceration and upper GI hemorrhage, uncontrolled hypertension, congestive heart failure, hyperkalemia, pregnancy, lactation and malignant tumors.
Randomization and treatment
A total of 60 subjects with symptoms of mild to moderate OA were selected and recruited into the study. Each subject was randomly assigned to the treatment group or placebo group using a randomization table generated using validated computer software CODE; IDV, Gauting, Germany. The randomization codes were secured confidential by the clinical trial pharmacist and statistician. Thirty subjects were allocated each into placebo and Aflapin groups. The subjects in Aflapin group received 50 mg of encapsulated Aflapin® twice daily, whereas, the subjects in the placebo group received two capsules having similar organoleptic properties including weight, taste, color, odor and feel. Each subject filled a questionnaire, providing details regarding demographics, medical history and nutritional status, at the baseline evaluation and during each follow-up evaluation on days 5, 15 and 30.
Assessments
Functional disability was assessed at baseline and at all follow-up visits (days 5, 15 and 30) by the investigators. Pain, stiffness and physical function were assessed using Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [22], LFI [21] and VAS [23] scores. The pain, stiffness and function subscales of the WOMAC were normalized to a scale of 0 to 100 units [24]. Analyses of these end-points were based upon the time-weighted average change from baseline over 30 days.
For assessment of safety of Aflapin®, several parameters were evaluated in serum, urine and whole blood of all subjects at each visit of the study duration (Table 1). Serum biochemical parameters and hematological parameters were measured using an automated analyzer (HumaStar 300) and a hematological counter (Humacount, Human, Wiesbaden, Germany). The urine analysis was carried out using UroColor™10 Dip Sticks and Urometer 600 (Standard Diagnostics, Kyonggi-do, Korea) and by sediment analysis using microscopy.
Rescue medication
Subjects were prescribed 400 mg ibuprofen tablets (maximum 400 mg thrice daily; total 1,200 mg) as rescue analgesia during the study based on pain intensity reported to the study physician by some subjects. Those subjects were advised not to take the rescue medicine at least 3 days before each evaluation. No other pain relieving interventions were allowed during the study period.
Statistical analysis
Detailed statistical analyses were performed using SAS software to evaluate the efficacy of Aflapin in comparison with the placebo group in terms of improvement in pain and physical function scores at baseline and on days 5, 15 and 30 of treatment. Wilcoxon's signed-rank test was used for inter group and intra-group comparisons of pain scores. Pair-wise changes were examined by carrying out least significant difference (LSD) test for all possible pairs. The significance of the effects of the treatment groups was compared by using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison tests. Results with P<0.05 are considered statistically significant. This is a two-arm (Aflapin and placebo), randomized, double-blind, placebo-controlled, single-centre trial conducted over 30 days. The trial's primary objective was to validate the efficacy of Aflapin on reduction of pain, joint stiffness and improvement in physical function in subjects with osteoarthritis of knee.
Parameters tested in Serum, urine and whole blood samples
| Biochemical Parameters |
| Blood sugar |
| Alkaline phosphatase |
| SGOT |
| SGPT |
| Total Bilirubin |
| Direct Bilirubin |
| CK Nac |
| Creatinine |
| Total Protein |
| Triglycerides |
| Cholesterol |
| HDL, LDL |
| Urea |
| Hematology |
| Total Leukocyte count |
| Total RBC count |
| Hemoglobin % |
| Mean Corpuscular volume (MCV) |
| Mean corpuscular hemoglobin (MCH) |
| Mean corpuscular hemoglobin Concentration |
| Platelet count |
| Differential count (DC) |
| Urine Analysis |
| Blood |
| Bilirubin, Urobilinogen |
| Ketone |
| Protein |
| Nitrite |
| Glucose |
| pH, Specific gravity |
| Leucocytes |
| Pus cells, Epithelial cells, Crystals |
Results
Baseline characteristics
The subjects were randomly distributed into two groups and the descriptive statistics comparing demographic variables, baseline disease characteristics and baseline outcome measures (LFI, VAS, WOMAC pain, function and stiffness sub-scores) are provided in Table 2. The demographic variables, disease-related and baseline outcome parameters of two groups, one receiving Aflapin® 100 mg/day (n=30) and the other receiving placebo (n=29) did not differ significantly at baseline.
Characteristics of patients in study groups.
| Characteristics | Placebo (n = 29) | 100 mg/day Aflapin® (n = 30) |
|---|---|---|
| Sex (male/female; n) | 11/18 | 11/19 |
| Age (years) | 55.3 ± 8.8 | 53.2 ± 6.5 |
| Body weight (kg) | 59.7 ± 10.5 | 61.9 ± 10.9 |
| Body mass index (kg/m2) | 24.9 ± 2.6 | 25.7 ± 3.3 |
| Visual analog score | 47.6 ± 9.7 | 48.0 ± 6.0 |
| Lequesne's Functional Index | 12.5 ± 3.4 | 12.8 ± 3.7 |
| WOMAC score | ||
| Pain subscale | 45.9 ± 10.5 | 47.8 ± 12.4 |
| Stiffness subscale | 37.5 ± 14.9 | 38.8 ± 13.3 |
| Function subscale | 40.6 ± 9.5 | 41.1 ± 11.8 |
Clinical efficacy
The data regarding the normalized pain and function scores are summarized in Table 3. At the end of the study, significant reductions in pain and function scores were observed in treatment group supplemented with 100 mg/day of Aflapin when compared to either baseline or placebo.
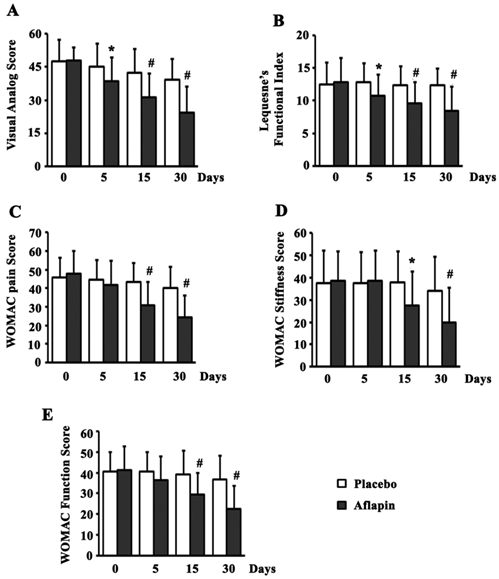
Significant (p<0.05) reduction in all the pain scores was observed in the Aflapin group by day 30, when compared to the placebo group. In comparison with placebo, supplementation of Aflapin for 30 days conferred 37.6, 32.0, 40.1, 41.3 and 38.8 percent reductions in VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC function scores, respectively. Interestingly, significant (p<0.05) reductions in VAS and LFI scores were also observed in Aflapin group over placebo by day 5. Aflapin supplementation showed 14.8 and 16.3 percent better reduction in VAS and LFI scores respectively over placebo by 5th day. Compared to the placebo group, the reductions in WOMAC scores were not significant after 5 days of treatment. Aflapin supplementation for 30 days afforded highly significant (p<0.001) reductions in all the pain scores exhibiting 49.1, 34.4, 49.5, 48.4 and 45.2 percent reduction, in VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC function scores, respectively, when compared to the baseline. However, significant (p<0.05) reductions were observed in VAS, WOMAC pain and WOMAC function scores in placebo group when compared to the base line and the magnitude of the reductions are 17.6, 12.0 and 9.24 percent respectively; which are small in comparison with those of the Aflapin group (figure 2).
Normalized pain and function scores.
| Parameter and treatment | Baseline mean ± SD | Day-30 mean ± SD | p value (vs. baseline) | p value (vs. placebo) |
|---|---|---|---|---|
| Visual analogue scale score | ||||
| Placebo (n=29) | 47.6 ± 9.7 | 39.3 ± 9.5 | <0.0001 | NA |
| Aflapin 100 mg/day (n=30) | 48.0 ± 6.0 | 24.5 ± 11.9 | <0.0001 | <0.0001 |
| Lequesne's Functional Index | ||||
| Placebo (n=29) | 12.5 ± 3.4 | 12.4 ± 2.6 | 0.7646 | NA |
| Aflapin 100 mg/day (n=30) | 12.8 ± 3.7 | 8.4 ± 3.8 | <0.0001 | <0.0001 |
| WOMAC pain subscale | ||||
| Placebo (n=29) | 45.9 ± 10.5 | 40.3 ± 11.4 | 0.001 | NA |
| Aflapin 100 mg/day (n=30) | 47.8 ± 12.4 | 24.2 ± 12.0 | <0.0001 | <0.0001 |
| WOMAC stiffness subscale | ||||
| Placebo (n=29) | 37.5 ± 14.9 | 34.1 ± 15.6 | 0.2024 | NA |
| Aflapin 100 mg/day (n=30) | 38.8 ± 13.3 | 20.0 ± 15.6 | <0.0001 | 0.0014 |
| WOMAC function subscale | ||||
| Placebo (n=29) | 40.6 ± 9.5 | 36.8 ± 11.5 | 0.0029 | NA |
| Aflapin 100 mg/day (n=30) | 41.1 ± 11.8 | 22.5 ± 11.1 | <0.0001 | <0.0001 |
NA, not applicable; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
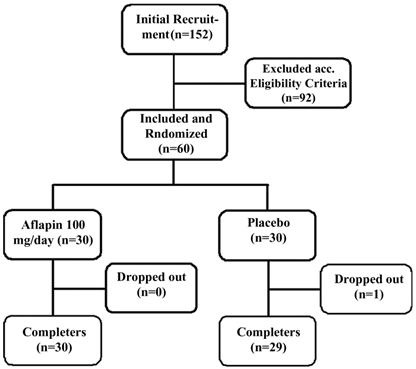
Flow chart of the subjects who participated in the clinical trial. Evaluations of physical activity and pain scores, serum biochemistry, hematology, urine biochemistry and pro-inflammatory biomarkers were done at baseline (day 0) and on days 5, 15 and 30 during follow up.
Pain, Function and stiffness scores. Presented are the mean scores for (a) visual analog scale, (b) Lequesne's Functional Index, (c) Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)-pain, (d) WOMAC-stiffness, and (e) WOMAC-function in placebo and 100 mg/day Aflapin® groups at different time points day 0, day 5, day 15 and day 30. Each bar represents mean ± standard deviation. In comparison with placebo the mean scores in the treatment groups were tested for significance using Wilcoxon's rank-sum test; * p < 0.05 and # p < 0.01.
Biochemical evaluations
As a part of the safety evaluation, laboratory tests were performed for assessment of different biochemical parameters (in serum and urine) and hematological parameters. The repeated measure ANOVA was used to compare the values at different evaluations over the 30 days period with those of baseline. Statistical analyses of these parameters did not indicate any significant changes. Although minor changes were observed in some of the parameters, they remained within the normal laboratory range. Similarly, no significant changes in hematological and urinary parameters were observed in the active treatment groups when compared to the placebo (data not shown).
Adverse Events and Dropouts
During the course of the 30-day study, no major adverse events were reported. However, nausea and headache were reported as minor adverse events by two subjects during the study; one each from placebo and Aflapin supplemented groups.
One subject from placebo groups was dropped out from the study due to un-availability for the follow up evaluations.
Discussion
The primary objective of conducting the present study was to substantiate the observation that Aflapin, a novel Boswellia extract reduces clinical symptoms of osteoarthritis, pain, physical discomfort. Boswellia serrata is an ancient Indian medicinal plant, and the gum resin of this plant has long been known for anti-inflammatory, anti-arthritic and analgesic properties [9,10]. Earlier studies indicate that 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA) is the most active principle present in the Boswellia extracts, which mainly contributes the anti-inflammatory activities of this herbal extract by inhibiting 5-lipoxygenase activity [11,12].
To date, the anti-inflammatory and anti-arthritic efficacy of different forms of Boswellia extracts have been established in various models either in vitro or in vivo or in clinical studies [13-16,19,25-30]. However, studies indicate that upon oral administration, Boswellia extracts exhibit poor intestinal absorption of AKBA and poor bioavailability which limits its anti-inflammatory efficacy [31,32]. Aflapin is a novel synergistic composition, which contains B. serrata extract enriched to 20% AKBA and B. serrata non-volatile oil (PCT/IN2009/000505). In a recent communication Sengupta et al [17] have reported that Aflapin® provides 51.78% more bioavailable concentration of systemic AKBA after a single dose oral administration in comparison with 30% AKBA enriched Boswellia extract (5-Loxin®). In corroboration, it was observed in a recent double blind placebo controlled study that Aflapin provides significantly better improvements in clinical symptoms in OA subjects when compared with 30% AKBA enriched Boswellia extract (5-Loxin®) [19]. The present 30-day double blind, placebo controlled clinical study was designed with two approaches; (i) to reassess the anti-arthritic efficacy of Aflapin and (ii) to evaluate the early onset of action of Aflapin in pain reduction and improvement of physical function in OA subjects.
The present study demonstrates the potential of Aflapin in alleviating pain, joint stiffness and improving physical functions in OA subjects (Figure 2). Pain, stiffness of joints, reduced joint movement and physical discomfort are the major clinical manifestations of OA [24,29,30]. In comparison with the placebo, at the end of the study, the Aflapin supplemented group showed statistically significant improvements in all pain scores including VAS, LFI, WOMAC pain, WOMAC stiffness and WOMAC function scores (Figure 2). Aflapin provided significant reductions in pain scores of VAS and LFI in as early as 5 days. Whereas, in the previous study Aflapin demonstrated significant relief from joint pain and physical discomfort in OA subjects after 7 days of treatment [19]. Together, these findings clearly suggest that Aflapin confers quick and significant pain relief, improvement in physical ability and quality of life in OA subjects.
Therapeutic efficacy and fast action of Aflapine can be attributed to its role in intervening the cellular and molecular mechanisms associated with the pathologic processes of OA. Earlier we have demonstrated multiple beneficial effects of Aflapin over 5-Loxin; (1), better anti-inflammatory efficacy of Aflapin through inhibiting 5-lipoxygenase enzyme activity, and inhibiting TNFα production; (2), provides significant protection from damaging action of IL-1β by increasing chondrocytes proliferation and increasing synthesis of cartilage matrix substances such as collagen and glycosaminoglycans in human primary chondrocytes; (3), Aflapin also inhibits MMP3 production in TNFα induced human chondrocytes [17].
Overall, the data demonstrate the efficacy of Aflapin in pain management, improving physical function, quality of life and joint health. Presumably, the pleotropic beneficial effects of Aflapin might provide potential anti-osteoarthritic efficacy, which helps improving joint health in OA subjects [17,19,25].
In corroboration with the previous studies [19,25], the present investigation does not show any major changes in the hematological parameters, serum biochemical parameters and in urine analysis in Aflapin supplemented subjects in comparison with placebo. In addition, no major adverse effect has been reported by the subjects included in Aflapin group. Taken together, these observations further demonstrate and substantiate the anti-osteoarthritic potential of Aflapin.
Conclusion
In summary, the present study validates the potential anti-OA efficacy and safety of Aflapin. In addition the present study also establishes the fast onset of therapeutic action of Aflapin® in OA subjects. Aflapin significantly improves joint function and relieves pain at as early as 5 days of treatment. This study bears potential promise in favor of Aflapin as a useful alternative therapeutic strategy for the management of OA in humans.
Abbreviations
AKBA: 3-O-acetyl-11-keto-beta-boswellic acid; ANOVA: analysis of variance; ASRAM: Alluri Sitarama Raju Academy of Medical Sciences; BMI: Body Mass Index; LFI: Lequesne's Functional Index; NSAID: nonsteroidal anti-inflammatory drug; NU: normalized units; OA: osteoarthritis; VAS: visual analog scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Acknowledgements
The authors sincerely thank Sri G Ganga Raju, Chairman; Mr. G Rama Raju, Director; and Mr. B. Kiran, CEO of Laila Group of Industries, India for their generous support and encouragements. This study is funded by Laila Impex R&D Center, India.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42:1-9
2. Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635-646
3. Karen WB. et al. Medical management of osteoarthritis. BMJ. 2000;321:936-940
4. Bos SD, Slagboon PE, Meulenbelt I. New insights into osteoarthritis: early developmental features of an aging related disease. Curr Opin Rheumatol. 2008;20:553-559
5. Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B, Bijlsma JW, Cluzeau F, Cooper C, Dieppe PA, Günther KP, Hauselmann HJ, Herrero-Beaumont G, Kaklamanis PM, Leeb B, Lequesne M, Lohmander S, Mazieres B, Mola EM, Pavelka K, Serni U, Swoboda B, Verbruggen AA, Weseloh G, Zimmermann-Gorska I. EULAR recommendations for the management of osteoarthritis: report of task force standing committee for International Clinical Studies including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2000;59:936-944
6. Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am J Med. 1998;105:31S-38S
7. Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug associated gastrointestinal injury. Am J Med. 1998;104:23S-29S
8. Wright JM. Double-edged sword of COX-2 selective NSAIDs. CMAJ. 2002;167:1131-1137
9. Singh GB, Atal CK. Pharmacology of an extract of salai guggal ex-Boswellia serrata, a new non-steroidal anti-inflammatory agent. Agents Actions. 1986;18:407-412
10. Ethan B, Heather B, Theresa DH, Ivo F, Sadaf H, Jens H, David S, Catherine U. Boswellia: An evidence-based systematic review by the natural standard research collaboration. J Herbal Pharmacother. 2004;4:63-83
11. Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HPT. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992;26:1143-1146
12. Sailer ER, Subramanian LR, Rall B, Hoernlein RF, Ammon HPT, Safayhi H. Acetyl-11-keto-β-boswellic acid (AKBA): structure requirements or binding and 5-lipoxygenase inhibitory activity. Br J Pharmacol. 1996;117:615-618
13. Joos S, Rosemann T, Szecsenyi J, Hahn EG, Willich SN, Brinkhaus B. Use of complementary and alternative medicine in Germany- a survey of subjects with inflammatory bowel disease. BMC Complement Altern Med. 2006;6:19
14. Anthoni C, Laukoetter MG, Rijcken E, Vowinkel T, Mennigen R, Muller S, Senninger N, Russell J, Jauch J, Bergmann J, Granger DN, Krieglstein CF. Mechanisms underlying the anti-inflammatory actions of boswellic acid derivatives in experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1131-G1137
15. Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke R, Safayhi H, Ammon HP. Effects of gum resin of Boswellia serrata in subjects with chronic colitis. Planta Med. 2001;67:391-395
16. Kimmatkar N, Thawani V, Hingorani L, Khiyani R. Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee: a randomized double blind placebo controlled trial. Phytomedicine. 2003;10:3-7
17. Sengupta K, Kolla JN, Krishnaraju AV, Yalamanchili N, Rao CV, Golakoti T, Raychaudhuri S, Raychaudhuri SP. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011;354:189-197
18. Krishnaraju AV, Sundararaju D, Vamsikrishna U, Suryachandra R, Machiraju G, Sengupta K, Trimurtulu G. Safety and toxicological evaluation of Aflapin®: A novel Boswellia-derived anti-inflammatory product. Toxicology Mechanisms and Methods. 2010;20:556-563
19. Sengupta K, Krishnaraju AV, Vishal AA, Mishra A, Golakoti T, Sarma KVS, Raychaudhuri SK, Raychaudhuri SP. Comparative Efficacy and Tolerability of 5-Loxin® and Aflapin® Against Osteoarthritis of the Knee: A Double Blind, Randomized, Placebo Controlled Clinical Study. Int J Med Sci. 2010;7(6):366-377
20. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, Moskowitz RW, Schnitzer TJ. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology. Arthritis Rheum. 1995;38:1541-1546
21. Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee validation-value in comparison with other assessment tests. Scand J Rheumatology. 1987;65:85-89
22. Bellamy N, Buchnan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to anti-rheumatic drug therapy in subjects with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833-1840
23. Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1-31
24. Bellamy N, Bell MJ, Goldsmith CH, Pericak D, Walker V, Raynauld JP, Torrance GW, Tugwell P, Polisson R. The effectiveness of hylan G-F 20 in subjects with knee osteoarthritis: an application of two sets of response criteria developed by the OARSI and one set developed by OMERACT-OARSI. Osteoarthritis Cartilage. 2005;13:104-110
25. Sengupta K, Krishnaraju AV, Satish AR, Mishra S, Golakoti T, Sarma KVS, Dey D, Raychaudhuri SP. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin® for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10:R85
26. Syrovets T, Buchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J Immunol. 2005;174:498-506
27. Gayathri B, Manjula N, Vinaykumar KS, Lakshmi BS, Balakrishnan A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFα, IL-1β, NO and MAP kinases. Int Immunopharmacol. 2007;7:473-482
28. Roy S, Khanna S, Shah H, Rink C, Phillips C, Preuss HG, Subbaraju GV, Trimurtulu G, Krishnaraju AV, Bagchi M, Sen CK. Human genome screen to identify the genetic basis of the anti-inflammatory effects of Boswellia in micro vascular endothelial cells. DNA Cell Biol. 2005;24:244-255
29. Roy S, Khanna S, Krishnaraju AV, Subbaraju GV, Yasmin T, Bagchi D, Sen CK. Regulation of vascular responses to inflammation: Inducible matrix metalloproteinase-3 expression in human microvascular endothelial cells is sensitive to anti-inflammatory Boswellia. Antioxid Redox Signal. 2006;3:653-660
30. Sengupta K, Golakoti T, Marasetti A, Tummala T, Ravada S, Krishnaraju A, Siba P Raychaudhuri SP. 30% 3-O-acetyl-11-keto-β-boswellic acid inhibits TNFα production and blocks MAPK/NFκB activation in lipopolysaccharide induced THP-1 human monocytes. J Food Lipids. 2009;16:325-344
31. Kruger P, Daneshfar R, Eckert GP, Klein J, Volmer DA, Bahr U, Muller WE, Karas M, Schubert-Zsilavecz M, Abdel-Tawab M. Metabolism of Boswellic Acids in Vitro and in Vivo. Drug Metabol and Disposition. 2008;36:1135-1142
32. Kruger P, Kanzer J, Hummel J, Fricker G, Schubert-Zsilavecz M, Abdel-Tawab M. Permeation of Boswellia extract in the Caco-2 model and possible interactions of its constituents KBA and AKBA with OATP1B3 and MRP2. Eur J Pharm Sc. 2009;36:275-284
Author contact
![]() Corresponding author: Siba P Raychaudhuri, email sraychaudhuriedu
Corresponding author: Siba P Raychaudhuri, email sraychaudhuriedu

 Global reach, higher impact
Global reach, higher impact