3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(6):501-509. doi:10.7150/ijms.8.501 This issue Cite
Research Paper
Oral Rehydration Therapy for Preoperative Fluid and Electrolyte Management
1. School of Nutrition & Dietetics, Kanagawa University of Human Services, Yokosuka, Kanagawa 238-8522, Japan
2. Department of Anesthesiology, Kanagawa Cancer Center, Yokohama 241-0815, Japan
Received 2011-6-26; Accepted 2011-8-19; Published 2011-8-25
Abstract
Aim: Preoperative fluid and electrolyte management is usually performed by intravenous therapy. We investigated the safety and effectiveness of oral rehydration therapy (ORT) for preoperative fluid and electrolyte management of surgical patients.
Methods: The study consisted of two studies, designed as a prospective observational study. In a pilot study, 20 surgical patients consumed 1000 mL of an oral rehydration solution (ORS) until 2 h before induction of general anesthesia. Parameters such as serum electrolyte concentrations, fractional excretion of sodium (FENa) as an index of renal blood flow, volume of esophageal-pharyngeal fluid and gastric fluid (EPGF), and patient satisfaction with ORT were assessed. In a follow-up study to assess the safety of ORT, 1078 surgical patients, who consumed ORS until 2 h before induction of general anesthesia, were assessed.
Results: In the pilot study, water, electrolytes, and carbohydrate were effectively and safely supplied by ORT. The FENa value was increased at 2 h following ORT. The volume of EPGF collected following the induction of anesthesia was 5.3±5.6 mL. In the follow-up study, a small amount of vomiting occurred in one patient, and no aspiration occurred in the patients.
Conclusion: These results suggest that ORT is a safe and effective therapy for the preoperative fluid and electrolyte management of selected surgical patients.
Keywords: Oral rehydration therapy, preoperative fluid and electrolytes, oral rehydration solution
Introduction
Preoperative fasting beginning the day before surgery has been standard practice to prevent aspiration pneumonia associated with general anesthesia [1]; thus before surgery, the patients are inevitably exposed to dry month and hunger. In Japan, preoperative fluid and electrolyte management is usually performed by intravenous therapy, and the fasting time prior to surgery seems to be longer than in other countries [2]. However, due to a lack of sufficient scientific evidence [1], the period of preoperative fasting has recently been reevaluated, and societies of anesthesiology in the United States and most European countries have revised the practice guidelines for preoperative fasting so that the oral intake of clear fluids may be permissible up to 2-3 h before surgery in selected surgical patients (excluding those in whom delayed gastric emptying is suspected) [3]. In addition, an approach for minimizing surgery-related stress and reducing subsequent complications has been introduced by Fearon et al. as the “enhanced recovery after surgery” (ERAS) protocol, addressing the disadvantage of preoperative fasting [4]. As reported by Nygren et al., preoperative oral provision of carbohydrates and fluids helps to alleviate anxiety of patients and also reduces the dry mouth and feeling of hunger caused by preoperative fasting [5]. Also, carbohydrate loading before surgery helps reduce postoperative insulin resistance [6]. In April 2007, our institution began using an oral rehydration solution (ORS) as the clear fluid for oral rehydration therapy (ORT) [7]. The ORS is one of the selections for dehydration treatment recommended by the World Health Organization (WHO) to supply water, electrolytes, and carbohydrates as comparable to intravenous therapy [8−10] and has recently gained widespread acceptance and is now preferred in the United States and in European countries. In the pilot study, we investigated the safety and effectiveness of ORT for the preoperative fluid and electrolyte management of patients receiving general anesthesia before breast surgery, and in the follow-up study on the safety of ORT, we assessed the safety of ORT in 1078 surgical patients who were treated with ORT during the12 months after the pilot study.
Methods
The study, designed as a prospective observation study, was approved by the institutional review board of the study institution (Kanagawa Cancer Center, Japan) and was conducted in accordance with the Declaration of Helsinki. Voluntary written informed consent was obtained from all subjects enrolled in the study.
In a pilot study, 20 patients who underwent breast surgery were enrolled in the study. The patients were those with physical status classification I or II of the American Society of Anesthesiologists (ASA), who were scheduled to enter the operating room at 13:00 for breast surgery. Patients who were unable to take food by mouth, patients who had previously received gastroesophageal surgery, patients with a body weight of 40 kg or less or 70 kg or more, patients with reduced creatinine clearance (80 mL/min or less), and patients with abnormal glucose tolerance (fasting blood glucose level, 120 mg/dL or more) were excluded from the study. The ORS containing water, glucose, and electrolytes, packaged in a 500-mL plastic bottle (OS-1, classified as a food in Japan, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan), was used in the study. Its composition is shown in Table 1. Patients consumed a standard diet at 18:00 on the day before surgery and fasted thereafter (with water permitted until 24:00). On the day of surgery, the patients drank 1000 mL of the ORS from a bottle from 8:00 to 11:00 at a volume of 333 mL/h. The patients were not premedicated and walked into the operating room at 13:00. Then, anesthesia was induced with propofol (1.5 mg/kg), fentanyl citrate (2 μ/kg), and vecuronium bromide (0.1 mg/kg); a laryngeal mask (Proseal #3, Laryngeal Mask Company, Henley-on-Thames, UK) was used to secure the airway. After the airway was secured, systemic anesthesia was maintained with propofol at 4 mg/kg per h. Blood, urine, and esophageal-pharyngeal fluid and gastric fluid (EPGF) samples were obtained within 3 min after the induction of anesthesia, and the volume of intravenous solution administered during that period was less than 10 mL. To sample EPGF, after induction of anesthesia, a gastric tube (14 Fr, Termo Co., Ltd., Tokyo, Japan) was inserted 75 cm from the tip of the drain tube of the laryngeal mask to sample EPGF. The tube was then pulled back to 45 cm from the tip of the drain tube while fluid was aspirated with a 50-mL catheter syringe GA (Nipro Corporation, Tokyo, Japan) under negative pressure in a face-up position. This procedure was repeated three times, and the gastric tube was then pulled back into the pharynx to sample EPGF. Sampling of EPGF was conducted by the same person.
Composition of oral rehydration solution
| Oral rehydration solution (OS-1) | ||
|---|---|---|
| Volume (mL) | 500 | |
| Energy (kcal) Carbohydrate (%) | 50 2.5 (glucose 1.8) | |
| Electrolytes (mEq/L) | ||
| Sodium (Na+) | 50 | |
| Potassium (K+) | 20 | |
| Magnesium (Mg2+) | 2 | |
| Lactate- | 31 | |
| Chloride (Cl-) | 50 | |
| Phosphorus (mmol/L) | 2 | |
| pH | 3.9 | |
| Osmolarity | Approx. 270 mOsm/L | |
With regard to the safety of ORT, the rates of occurrence of vomiting and aspiration at the time of induction of anesthesia were investigated (in 20 patients). Volumes of EPGF obtained following induction of anesthesia were measured. Vital signs were measured before and at 1 and 2 h after ingestion of the ORS was completed. Blood pressure and pulse rate were measured at the right upper arm bound with a cuff by a bed-side monitor (BSM-2301, Nihon Koden Corporation, Tokyo, Japan). Body temperature was measured at the right axilla by an electronic thermometer (ET-C202P01, Termo Corporation, Tokyo, Japan).
With regard to the efficacy of ORT, the changes in serum electrolyte (sodium, potassium, and chloride), glucose, and hematocrit values following rehydration with the ORS was evaluated before and 2 h after the end of ORS consumption. The samples were analyzed immediately after they were obtained. The electrolyte concentrations in blood, urine, and EPGF as well as serum glucose were measured using an automatic analyzer (Hitachi 7170S, Hitachi High-Technologies Corporation, Tokyo, Japan); blood cell counts were determined using an automatic blood cell analyzer (Sysmex XE-2100, Sysmex TMC, Kobe, Japan); and pH values were measured with a pH meter (B-211, Horiba, Ltd., Kyoto, Japan). To estimate renal blood flow, the fractional excretion of sodium (FENa) and the change in FENa (∆FENa) following rehydration were evaluated. The FENa was calculated by the following equation; FENa= (urinary sodium concentration × serum creatinine value/serum sodium concentration × urinary creatinine value) × 100.
With regard to the assessment of patient satisfaction, the incidence rates of feeling hunger, dry mouth, and a feeling of restriction, which were determined by using a questionnaire, were investigated to assess the patients' satisfaction with the treatment.
Descriptive statistics (the number of patients, mean value, standard deviation, maximum value, top quartile, median value, bottom quartile, and minimum value) were obtained for serum electrolytes (sodium, potassium, and chloride), serum glucose, serum creatinine, hematocrit, vital signs, preoperative urine volume, urinary sodium, and urinary creatinine. Vital signs were analyzed using the repeated-measures analysis of variance and the Dunnett test (two-sided at α=0.05). Blood and urine values and FENa values before and after treatment were analyzed for differences using the t-test (two-sided at α=0.05).
In the follow-up study to assess the safety of ORT, 1078 surgical patients who received ORT before induction of general anesthesia during 12 months (August 2007 to August 2008) after the pilot study were assessed. The patients were those with physical status classification I or II of American Society of Anesthesiologists (ASA) who were judged appropriate for ORT by the attending physician and agreed to receive the ORT. Inclusion and exclusion criteria were the same as in the pilot study. The patients, who did not agree to receive the ORT, received intravenous therapy instead. The oral rehydration solution given to the patients was the same as that used in the pilot study. Following a meal and after 19:00 on the day before surgery, the patients were given three bottles of the study solution (500 mL × 3 bottles) and allowed to freely consume the solution until 2 h before entering the operating room for surgery, same as in the pilot study, but were instructed not to consume a large volume at a time (consume in a divided volume). The largest volume of consumption was set to be 1500 mL and the patients, if unable to consume at least 500 mL, received intravenous therapy. For patients with malignant gastric cancer, the timing of consumption was limited to up to 6 h before surgery because of the possibility of delayed gastric emptying. Bowel preparation such as using laxatives was not restricted during the study period. The patients were not premedicated and walked into the operating room. The method of anesthesia was not specified. For the safety assessment, the occurrence rates of vomiting and aspiration at the time of induction of anesthesia were investigated to assess the adverse events and adverse reactions associated with the therapy. Vomiting was defined as the reflux of gastric or esophageal content to oral cavity at the time of anesthesia induction. Aspiration was defined as the case in which the contents of vomiting are identical to the tracheal contents aspirated through endotracheal intubation.
Results
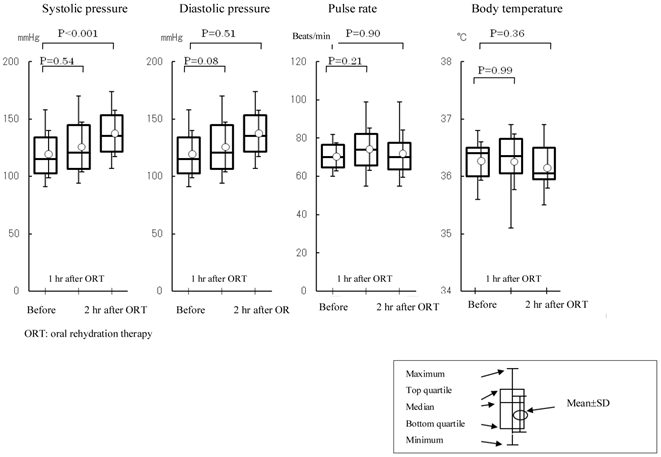
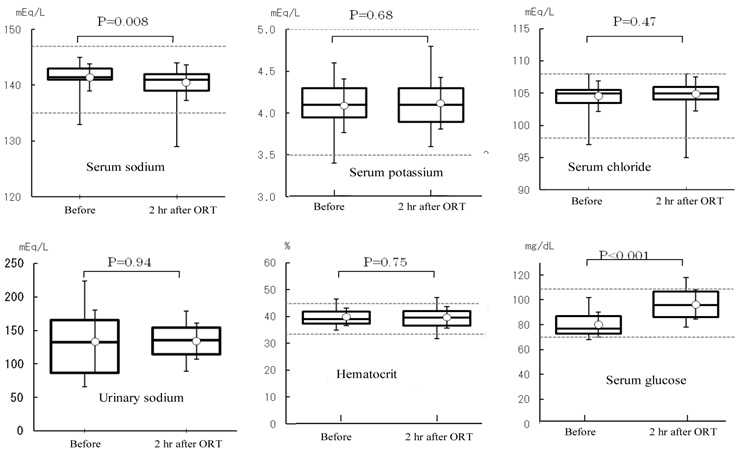
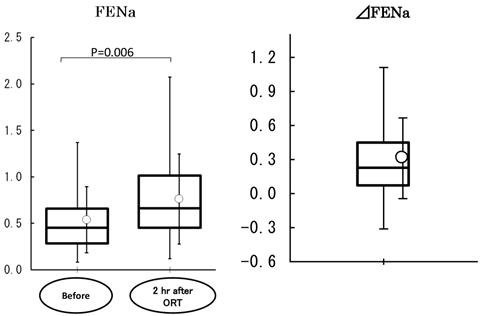
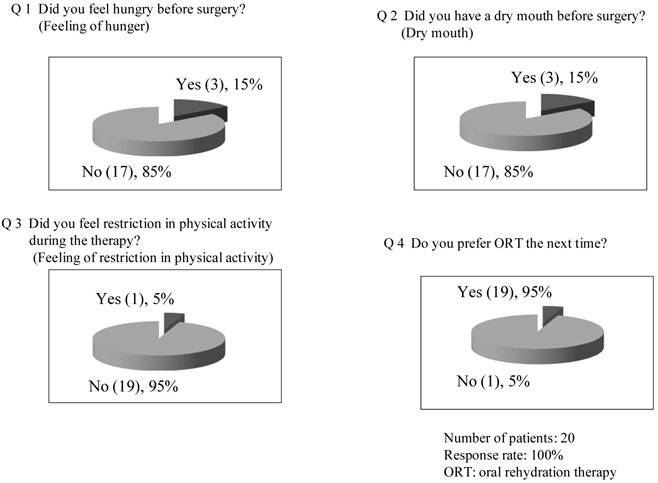
Pilot study: Twenty patients were registered in the study. Their baseline characteristics are shown in Table 2. Creatinine clearance value, measured as an index of renal function, was 99.9±18.4 mL/min (n=20). Vomiting and aspiration associated with the induction of general anesthesia were not observed. The volume of EPGF collected following induction of anesthesia was 5.3±5.6 mL (0.1±0.1 mL/kg). With regard to vital signs, blood pressure (diastolic), pulse rate, and body temperature were not changed following treatment. Systolic blood pressure showed an increase at 2 h after treatment (before anesthesia) (126±22 mmHg vs. 137±20 mmHg, P<0.001) (Figure 1). With ORT, no changes were observed in the serum concentrations of potassium and chloride and the hematocrit value (Figure 2). In contrast, the serum concentration of sodium was decreased within the normal limits (sodium: 135-147 mEq/l) established at the study institution, and no changes were observed in the urinary sodium concentrations. The blood glucose was increased within the normal limits (70-110 mg/dL) (Figure 2). FENa was increased at 2 hr following ORT (0.54±0.36 vs. 0.76±0.48, p=0.006), and ΔFENa showed a positive value (0.22±0.32) (Figure 3). The results of the questionnaire survey on patient satisfaction with the ORT are shown in Figure 4. Most of the patients (95%) replied that they would prefer ORT the next time.
Baseline characteristics of patients in the pilot study
| Number of patients | % | ||
|---|---|---|---|
| Patients | 20 women | ||
| Age (years) | 20−39 | 3 | 15.0 |
| 40−59 | 11 | 55.0 | |
| 60−79 | 6 | 30.0 | |
| 80 or more | 0 | 0.0 | |
| Body weight (kg) | Less than 50 | 4 | 20.0 |
| 50−59 | 11 | 55.0 | |
| 60−69 | 5 | 25.0 | |
| 70−79 | 0 | 0.0 | |
| 80 or more | 0 | 0.0 | |
| Height (cm) | Less than 150 | 2 | 10.0 |
| 150−159 | 14 | 70.0 | |
| 160−169 | 4 | 20.0 | |
| 170 or more | 0 | 0.0 | |
| Diagnosis | Breast cancer | 20 | 100 |
| Other | 0 | 0 | |
| ASA physical status classification | ASA I | 16 | 80.0 |
| ASA II | 4 | 20.0 | |
| Complications and past history | None | 15 | 75.0 |
| Present | 5 | 25.0 |
ASA: American Society of Anesthesiologists.
Changes in blood pressure, pulse rate, and body temperature. ORT: oral rehydration therapy. Values at each measurement time point were analyzed for changes over time by repeated measures analysis of variance (α=0.05). If differences from baseline value (i.e., changes over time) were significant, changes from the baseline value were analyzed by the Dunnett test (α=0.05). Systolic blood pressure showed an increase at 2 h after treatment (before anesthesia) (P<0.001).
Serum electrolyte (sodium, potassium, and chloride), urinary sodium, hematocrit, and serum glucose values. ORT: oral rehydration therapy. Values were analyzed by the t-test (α=0.05). Following treatment, serum sodium level was decreased within the normal ranges specified at the study hospital (P=0.008) and the glucose level was increased (P<0.001).
Fractional excretion of sodium (FENa) and the change in FENa (∆FENa) following rehydration (ORT). FENa= (urinary sodium concentration × serum creatinine value/serum sodium concentration × urinary creatinine value) × 100. ∆FENa: change in FENa following rehydration. Values were analyzed by the t-test. FENa was increased at 2 h following rehydration (P=0.006) and ∆FENa was positive.
Patient satisfaction (Q&A) with the therapy (ORT).
Follow-up study: A total of 1078 patients who received ORT before surgery during 12 months after the pilot study were evaluated. Primary diseases of these patients are shown in Table 3. Neurosurgical and colon surgery patients were not included in the study because the consents for patient enrolment in the study were not obtained from their attending physicians (because there are risks of a decrease in conscious level, paralysis, and an increase in intracranial pressure in neurosurgical patients as well as risks of preoperative reduction of intragastric tube pressure in colon surgery patients). In one patient (0.09%) with ASA III to whom the ORS was not considered appropriate, minor vomiting occurred after bag-valve-mask ventilation was performed. In female patients with malignant mammary tumor, 3 patients could not consume more than 500 mL of ORS because of taste preference and were treated with an intravenous therapy instead.
Primary diseases of patients in the follow-up study
| Primary diseases | Number of patients | Abnormality after induction of general anesthesia |
|---|---|---|
| Malignant mammary tumors | 237 | |
| Malignant head and neck tumors | 124 | |
| Malignant tumors of the stomach | 79 | |
| Malignant tumors of the respiratory systems | 171 | |
| Malignant tumors of the urinary and genital organs | 228 | Vomiting, 1 |
| Malignant gynecological tumors | 46 | |
| Malignant bone and soft tissue tumors | 122 | |
| Malignant tumors of the body surface | 27 | |
| Other | 44 | |
| Total | 1078 | 1 |
Period of follow-up assessment: Between August 2007 and August 2008.
Average age (years): 60.2 (18 to 92). Sex ratio: 507 men and 674 women.
Discussion
The ORT used in the present study has been recognized to be safe and clinically effective for the treatment of patients with cholera [9] and is considered to be an effective therapy for the treatment of dehydration and has attracted a great deal of interest in the United States and EU countries. Also, the use of oral rehydration solutions is recommended by the Centers for Disease Control and Prevention in the United States for the treatment of patients with mild-to-moderate dehydration [8]. The OS-1, which was used in the present study, is based on the concept of ORT as recommended by the World Health Organization [10], and its composition is based on the guidelines of the American Academy of Pediatrics [11]. In Japan, OS-1 has been approved as a food (classified as a food for special dietary use) by the Ministry of Health, Labour and Welfare of Japan, and has been shown to be effective for the provision of water and electrolytes in patients with dehydration as well as postoperative patients [12, 13]. Taking these advantages into consideration, we have been using ORT for the preoperative management of fluids and electrolytes in selected surgical patients in our hospital.
With regard to the safety of ORT following induction of anesthesia in the pilot study, there were no cases of aspiration or vomiting associated with induction of general anesthesia. A risk of aspiration has been reported to occur if the volume of gastric contents exceeds 200 mL at the time of anesthesia induction [3], but no patients were found to have a volume of gastric contents greater than 200 mL in the present study. With regard to vital signs, changes in blood pressure (diastolic), pulse rate, and body temperature were significant before and at 1 and 2 h after treatment, but systolic blood pressure showed an increase at 2 h after treatment. This increase was considered attributed to psychological pressure coupled with the time of entry to the operating room.
With regard to the effectiveness of ORT, the FENa was assessed as an index reflecting the effect on water supplementation. The FENa is a value that indicates the percentage of sodium filtered by the renal glomerular capillaries and may be a sensitive index of the renal blood flow in subjects with normal renal function such as those enrolled in the present study. If the renal blood flow volume decreases in response to a reduction in circulating blood volume in dehydration, the FENa value falls because the excretion of sodium is reduced to promote sodium retention [14]. In the present study, because of the effect of preoperative fasting from the evening before the day of surgery, many patients showed low FENa values, which rose in response to rehydration by administration of the study solutions. These observations can be interpreted to mean that many patients were dehydrated in the morning of the day of surgery as a result of preoperative fasting but rehydrated by ORT. Following rehydration, the serum chloride and potassium levels and hematocrit values showed no change, but the serum sodium level was decreased and glucose levels were increased. Considering that the urinary concentration of sodium did not change, the decrease in the serum sodium value is thought to be the result of dilution due to the effect of fluid supplementation by ORT. The blood glucose, which was lowered by fasting, increased with glucose intake within the normal limit. Consequently, it can be said that the results of this study confirm the effectiveness and safety of ORT to some extent.
Next, with regard to the questionnaire survey on patient satisfaction with ORT (the survey was conducted on the day after surgery), there were almost no complaints about dry mouth, feeling of hunger, and feeling of physical restriction before surgery, and it was judged that patient satisfaction with ORT was high in all questionnaire items, as commented by the patients that they would prefer ORT to an intravenous therapy the next time, too. However, since some complains were received in each questionnaire item (including the case of refusing to drink the fluid because of taste preference), the timing and volume of consumption and the taste and temperature of the fluid should be further improved so that all patients can pleasantly drink for fluid and electrolyte replenishment before surgery.
In the follow-up study of 1078 patients, vomiting occurred in one patient (0.09%) following the induction of general anesthesia, and no aspiration was observed. This patient, who was receiving an oxygen therapy at home for chronic obstructive respiratory disease, was in grade III of the ASA physical status classification and, basically, was not the patient appropriate for the ORT treatment, and it was probable that hyperinflation of the lung was always present in the patient, compressing abdominal organs and delaying the movement of stomach content downwards. Although aspiration was not observed in this patient, aspiration has been reported to be fatal in the ASA III or IV grade patients [15]. Therefore, when we use ORT, it seems requisite to strictly follow specific standard. On the basis of these findings observed in the follow-up patients, ORT can be judged to be safe and effective when used before anesthesia induction, although the conditions of use must be strictly followed. The eligibility standard for ORT at our hospital, which has been based on the results of this prospective observational study (pilot and follow-up study), is shown in Table 4, incorporated in our hospital manuals and known to our hospital personnel.
Finally, it may be true that the duration of preoperative fasting is still long in Japan as compared with that in the United States and EU countries, and intravenous therapy is more likely to be used in Japan [2]. One reason lies in that there are no authorized national guidelines for the practice of preoperative fasting in this country, and some sort of guidelines should be established. Looking at the guidelines in the United States or the European Union, no specific recommendations are given as to what kind of clear fluid should be use. The oral rehydration solution used in the present study has been confirmed to be more-or-less equal to the intravenous therapy in terms of the effect on the supplementation of water, electrolytes, and carbohydrates [8, 9]. We conclude that ORT is a safe and effective therapy for the preoperative fluid and electrolyte management of selected surgical patients.
Eligibility standard for oral rehydration therapy
| I. Patients eligible for oral rehydration therapy (receiving oral rehydration solution) |
| 1. Those who give an informed consent and have not been treated with prior medications 2. Those with physical status classification I or II of ASA* |
| II. Relative contraindications (patients may consume an oral rehydration solution if permitted by attending anesthesiologist) |
| 1. Those who are unable to understand instructions about the therapy (such as how to consume the oral rehydration solution). 2. Those who had previously received surgery of upper gastrointestinal tract, liver, biliary tract, or pancreas 3. Those who may have poor intestinal motility a) Severe obesity (BMI more than 28) b) Severe diabetes mellitus 4. Those who are at high risk of aspiration a) Those who have abnormality of recurrent lanryngeal nerve due to neck-and-head diseases b) Those who may have difficulty in endotracheal intubation and mask ventilation c) Those who need prior medication of sedatives d) Those aged more than 80 years |
| III. Absolute contraindications |
| 1. Those who are not permitted to take food by mouth |
| 2. Those who have gastrointestinal obstruction |
| 3. Those who have an increased cerebral pressure and consciousness disorder |
| 4. Those with physical status classification of ASA III or greater |
* American Society of Anesthesiologists.
Acknowledgements
This study was presented in part at the Japanese Society of Anaesthesiologists 53rd Annual Meeting, Sapporo, Japan (May 2007) and at the 23rd Japanese Society for Parenteral and Enteral Nutrition, Kyoto, Japan (February 2008).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. McIntyre JWR. Evolution of 20th century attitudes to prophylaxis of pulmonary aspiration during anaesthesia. Can J Anaesth. 1998;45:1024-30
2. Shime N, Ono A, Chihara E, Tanaka Y. Current practice of preoperative fasting: a nationwide survey in Japanese-teaching hospitals. J Anesth. 2005;19:187-92
3. Søreide E, Eriksson LI, Hirlekar G, Eriksson H, Henneberg SW, Sandin R, Raeder J. Pre-operative fasting guidelines: an update. Acta Anaesthesiol Scand. 2005;49:1041-7
4. Fearon KCH, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CHC, Lassen K, Nygren J, Hausel J, Soop M, Andersen J, Kehlet H. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466-77
5. Nygren J, Thorell A, Jacobsson H, Larsson S, Schnell PO, Hylén L, Ljungqvist O. Preoperative gastric emptying: effects of anxiety and oral carbohydrate administration. Ann Surg. 1995;222:728-34
6. Soop M, Nygren J, Myrenfors P, Thorell A, Ljungqvist O. Preoperative oral carbohydrate treatment attenuates immediate postoperative insulin resistance. Am J Physiol Endcriol Metab. 2001;280:E576-83
7. Taniguchi H, Sakaki T, Fujita H, Takamori M, Kawasaki R, Momiyama Y, Takano O, Shibata T, Goto T. Preoperative fluid and electrolyte management with oral rehydration therapy. J Anesth. 2009;23:222-9
8. Farthing MJG. Oral rehydration therapy. Pharmac Ther. 1994;64:477-92
9. Celeb KK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: Oral rehydration, maintenance, and nutritional therapy. Morb Mortl Wkly Rep (MMWR). 2003;52(No.RR-16):1-16
10. World Health Organization. A manual for the treatment of diarrhea; WHO/CDD/SER/80.2; Rev 2. WHO. 1990.
11. American Academy of Pediatrics. Committee on Nutrition. Use of oral fluid therapy and post-treatment feeding following enteritis in children in a developed country. Pediatrics. 1985;75:358-61
12. Nishi M, Okahisa T, Yano H, Sogame M, Kishi S, Tsuruo M, Sasaki K, Okada A, Mizuyama K, Yano S, Shimada H, Kyoda S, Tsuru T, Umezu T, Fujino M, Shirataka M. Effectiveness of OS-1 for water and electrolyte supplementation in dehydrated patients with infectious enteritis or the common cold-multicenter clinical study using commercially available mineral water as a control solution (in Japanese with English abstract). Yakuri to Chiryo (Jpn Pharmacol Ther). 2003;31:839-53
13. Goseki N, Hiranuma S, Yamazaki S, Maruyama M, Nakajima K, Gen T, Shirataka M. Oral rehydration solution for providing water and electrolytes following laparoscopic cholecystectomy and recovery of intestinal function. Hepato-Gastroenterology. 2007;54:2276-81
14. Irwin RS, Rippe JM. Irwin and Rippe's Intensive Care Medicine; 6th Edition. Philadelphia: Lippincott Williams and Wilkins. 2008:880-1
15. Engelhardt T, Webster NR. Pulmonary aspiration of gastric contents in anaesthesia. B J Anaesth. 1999;83:453-60
Author contact
![]() Corresponding author: Hideki Taniguchi, MD, School of Nutrition & Dietetics, Kanagawa University of Human Services, 1-10-1 Heiseicho, Yokosuka, Kanagawa 238-8522, Japan. Address e-mail: taniguchi-hdkac.jp
Corresponding author: Hideki Taniguchi, MD, School of Nutrition & Dietetics, Kanagawa University of Human Services, 1-10-1 Heiseicho, Yokosuka, Kanagawa 238-8522, Japan. Address e-mail: taniguchi-hdkac.jp

 Global reach, higher impact
Global reach, higher impact