Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(5):369-376. doi:10.7150/ijms.8.369 This issue Cite
Research Paper
The Association Among Lipoprotein-associated Phospholipase A2 Levels, Total Antioxidant Capacity and Arousal in Male Patients with OSA
1. Department of Pulmonary Medicine, Konya Education and Research Hospital, Konya/ Turkey.
2. Department of Cardiology, Meram Medical Faculty, Selcuk University, Konya, Turkey.
3. Department of Biochemistry, Meram Medical Faculty, Selcuk University, Konya, Turkey.
4. Department of Pulmonary Medicine, Meram Medical Faculty, Selcuk University, Konya, Turkey.
Received 2011-3-17; Accepted 2011-4-25; Published 2011-6-10
Abstract
Background: The mechanisms of the increased cardiac and vascular events in patients with OSA are not well understood. Arousal which is an important component of OSA was associated with increased sympathetic activation and electrocardiographic changes which prone to arrhythmias. We planned to examine the association among arousal, circulating Lp-PLA2 and total antioxidant capacity in male patients with OSA.
Methods: Fifty male patients with newly diagnosed OSA were enrolled the study. A full-night polysomnography was performed and arousal index was obtained. Lp-PLA2 concentrations were measured in serum samples with the PLAC Test. Total antioxidant capacity in patients was determined with Antioxidant Assay Kit.
Results: Arousal was positively correlated with LP-PLA2 levels (r=0.43, p=0.002) and was negatively correlated with total antioxidant capacity (r= -0.29, p=0.04). Elevated LP-PLA2 levels and decreased total antioxidant activities were found in the highest arousal quartile compared with the lowest and 2nd quartiles (p=0.02, p=0.05, respectively). LP-PLA2 was an independently predictor of arousal index in regression model (β=0.357, p=0.002)
Conclusions: This study demonstrated a moderate linear relationship between arousal and LP-PLA2 levels. Also, total antioxidant capacities were decreased in the higher arousal index. Based on the study result, the patients with higher arousal index may be prone to vascular events.
Keywords: Obstructive sleep apnea, Arousal, Lipoprotein-associated phospholipase A2, total antioxidant status, cardiovascular risk
Introduction
Obstructive sleep apnea (OSA) is independently related with increased risk for hypertension, ischemic stroke, and myocardial ischemia [1-3]. The mechanisms of the increased cardiac and vascular events in patients with OSA are not well understood. Arousal, in the absence of hypercapnia or hypoxia, has been reported to be associated with an acute increase in sympathetic activity [4]. Arousals from sleep are associated with acute surges in blood pressure and heart rate [5-7]. The repeated arousals from sleep that occur in OSA may contribute to the increased risk of developing hypertension [1, 8], with the mediating factor being the frequent, acute cardiovascular insults [9]. Arousal was also related with electrocardiographic changes at the RR, QT and PR intervals [10-12].
The measurement of circulating cardiovascular risk factors (including several novel markers of cardiovascular disease) enables a more accurate prediction of cardiovascular risk to be made, as there are clearly established relationships between levels of various circulating haemostatic risk factors and a subsequent cardiovascular event [13, 14]. In recent years, several epidemiology studies have showed an association among lipoprotein-associated phospholipase A2 (Lp-PLA2), a biomarker that may be viewed as a potential link between noxious effects of oxidized LDL cholesterol and elusive plaque vulnerability, cardiovascular and cerebrovascular events [15-21]. Increased oxidative stress and inflammation were also demonstrated in patients with OSA [22]. Although, the relation between arousal and cardiovascular changes was known, the association among circulating LP-PLA2, total antioxidant capacity and arousal has not been studied yet. We planned to examine the association between circulating cardiovascular risk markers and arousal in patients with OSA.
Methods
Patients who were referred to the Sleep laboratory for evaluation of sleep-disordered breathing between April 2009 and January 2010 were prospectively screened for the study. Fifty male patients with newly diagnosed OSA were enrolled to the study but women are not included in this study. OSA was defined as an apnea-hypopnea index (AHI) of ≥5 obstructive events per hour of sleep. The patients who did not meet exclusion criteria's were consecutively enrolled the study. Patients with hypertension, coronary artery disease, heart failure, a history of stroke, diabetes mellitus, chronic obstructive or restrictive pulmonary disease, chronic renal disease, dyslipidemias, pharmacologically treated depression, and tobacco use within the past 10 years were excluded from the study. In addition, nightshift workers and patients receiving medications or nutritional supplements were ineligible for the study. The Selcuk University Ethic Committee approved the study and written informed consent was obtained from all study participants.
Polysomnography
At least one full-night polysomnography (PSG) was performed by using Compumedics E-series Sleep System, (Compumedics, Melbourne, Australia). Electroencephalography (EEG), submental electromyography (EMG), leg EMG, electrooculography (EOG), and electrocardiography (ECG) recordings were obtained; air-flow was measured using both a nasal cannula (NC) and nasal thermistor, oxygen saturation (SaO2) was measured using a pulse oximeter, and chest and abdominal respiratory movements were monitored. A reduction in oxygen saturation to ≤ 4 or the occurrence of symptoms of physiologic awakening, following at least a 30% reduction in air flow for a minimum of 10 sec, was considered as hypopnea. Individuals with an apnea hypopnea index (AHI) >5 were diagnosed as OSAS and included in the study.
Determining of Arousals
An arousal was defined as an increase in EEG and/or EMG activity (frequency and amplitude) that varies significantly from the background activity defining the current sleep stage. An arousal was scored; during NREM sleep consisting of an abrupt EEG frequency shift (eg, alpha, theta, or frequencies >16 Hz, but not spindles) lasting at least 3 s and accompanied by at least 10 s of stable sleep. A REM arousal is characterized by similar EEG changes but should be accompanied by an increase in chin EMG that is at least 1 s in duration. The arousal index is the number of the arousals per hour of sleep [23].
Study Protocol
All subjects underwent attended nocturnal polysomnography in the sleep laboratory. Nocturnal polysomnography was performed as previously described. AHI was defined as the number of obstructive apnea plus hypopnea episodes per hour of sleep. Weight, height, waist and hip circumference and blood pressure of patients were measured. Other demographics such as diabetes mellitus, hypertension and smoking status were recorded. Body mass index was calculated with formula. The patients were divided into two groups according to median arousal index as above or below median. The demographic features and parameters of polysomnography test were expressed as to median arousal. Venous blood samples were drawn into serum separator clot activator tubes at 9:00 A.M. from patients after overnight fasting within 48 hours of polysomnography.
Lp-PLA2
Lp-PLA2 concentrations were measured in serum samples with the PLAC Test (diaDexus Inc, USA) reagent kit on UniCel DxC 800 Synchron Clinical System (Beckman Coulter, USA) automated clinical chemistry analyzer. The PLAC test is a turbidimetric immunoassay using two highly specific monoclonal antibodies against Lp-PLA2. Lp-PLA2 concentrations were given as ng/ml. Clinical and analytical sensitivities of the assay are 7 ng/ml and 4 ng/ml respectively. Reference intervals suggested by the reagent manufacturer are 120-342 ng/ml for females and 131-376 ng/ml for males. The assay is linear up to 500ng/ml without prior dilution. Intra-assay and inter-assay precisions are %2 and %1,6 ( Control 1: 143,9 ng/ml) and %1,6 and %0,8 (Control 2: 449,8) respectively. The patients were divided into four groups according to arousal's quartile. Arousal range of patients in the quartiles was defined as the lowest quartile: 1.8-8.4, 2nd quartile: 8.8-14.1, 3rd quartile: 14.4-20.5, the highest arousal quartile: 23.6-71.6. LP-PLA2 level of each arousal quartile was compared. LP-PLA2 level of > 200 ng/ml was accepted as an elevated LP-PLA2 [24].
Total Antioxidant Capacity
Total antioxidant capacity in patients was determined with Antioxidant Assay Kit (Cayman Chemical Company, USA). The assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS® (2,2'-azino-di-[3-ethylbenzthiazoline sulphonate]) to ABTS® · + by methmyoglobin. The capacity of the antioxidants in the sample to prevent ABTS oxidation is compared with that of Trolox, a water-soluble tocopherol analogue, and is quantified as mM Trolox equivalents. Samples containing antioxidants between 0,044-0,330 mM can be assayed without further dilution. Inter-assay and intra-assay values are %3 and %3,4 respectively
Statistical Analysis
Data were analyzed by using SPSS software version 13.0 (SPSS, Chicago, IL, USA) and were expressed as mean ± standard deviation. The seasonal distribution of the variables was analyzed with Kolmogorov-Smirnow test. Correlation analysis was carried out with Pearson's correlation test for normally distributed variables. Linear regression analysis was performed to determine the predictors of arousal index. Firstly, linear regression analysis was performed with enter method and later it was performed with stepwise method. Independent Student's t tests were used for comparing differences of parametric variables between two groups. The difference between nonparametric variables was tested by Mann Withney U test. Kruskal-Wallis H tests were used for comparing medians of continuous variables among quartiles of arousal. When statistically significant differences occurred, single posttest comparisons were performed by using the Mann-Whitney U test with Bonferroni correction for multiple comparisons. Differences in prevalence were tested by the nonparametric chi-square test. p value of < 0.05 was considered as statistically significant for all the tests.
Results
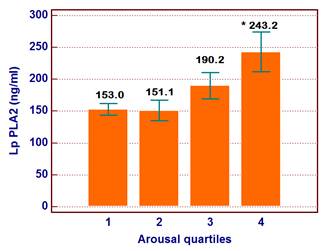
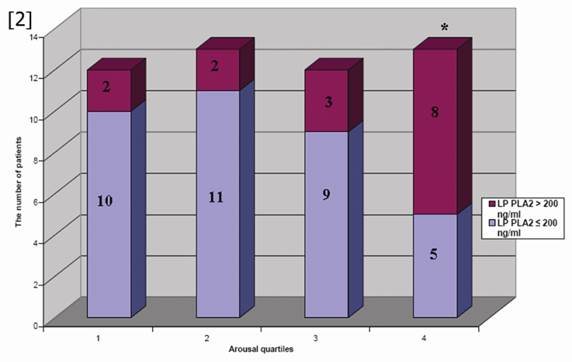
The ages of the participants were between 35-60 years (Mean±SD was 43.5±10.5 years). Patients were divided into two groups according to the median arousal value and patients' characteristics and laboratory findings were demonstrated in Table 1. LP-PLA2, total cholesterol and triglyceride levels were increased in patients above median arousal and TAC was reduced in these subjects. Other demographic and laboratory findings were comparable in two groups. According to the arousal quartile, the level of LP-PLA2 was increased in the highest arousal quartile compared to the lowest and 2nd quartile (p=0.02) (Figure 1). Thus, elevated LP-PLA2 values were originated from the highest arousal quartile. In addition, the number of patients with elevated LP-PLA2 (>200 ng/ml) was increased in the highest arousal quartile compared to the other quartiles (n=2, n=2, n=3 and n=8, p=0.035, respectively from the lowest arousal quartile to the highest arousal quartile) (Figure 2).
In the stepwise linear regression model, a LP-PLA2 level was independently associated with arousal (p=0.002). Linear regression analysis with enter method demonstrated that only LP-PLA2 levels were related with arousal (Table 2), but hsCRP, total antioxidant capacity, BMI, and age were not related with arousal (Table 3).
The study demonstrated a significant negative correlation between total antioxidant capacity and arousal (r= -0.29, p=0.04). Also, total antioxidant capacity was decreased in the group of below the median arousal index (p=0.05) (Table 1).
Correlation analysis between LP-PLA2 and other variables were demonstrated in Table 4.
It was demonstrated that LP PLA2 level was significantly increased in the highest arousal quartile. * p=0.02
The distribution of patients with elevated LP PLA2 levels was demonstrated according to the arousal quartiles in the figure. The most of patient with elevated LP PLA2 level were clustered in the highest arousal quartile. *p=0.035
Demographic features and laboratory findings according to the median value of arousals
| Below the median arousal (<14.25) n=25 | Above the median arousal (>14.25) n=25 | p | |
|---|---|---|---|
| Age | 41,9±10,6 | 44,1±10,2 | 0.26 |
| Smoker (n) | 9 | 14 | 0.35 |
| Hypertension (n)* | 3 | 1 | 0.45 |
| Diabetes mellitus (n) | 2 | 1 | 0.68 |
| BMI (kg/m2) | 30,1±3,9 | 28,8±4,6 | 0.12 |
| Waist / hip circumference ratio | 0,96±0 ,04 | 0,95±0,06 | 0.83 |
| Systolic Blood pressure (mmHg) | 126,1±11,5 | 124,6±10,2 | 0.64 |
| Diastolic blood pressure (mmHg) | 74,4±5,1 | 75,8±7,6 | 0.44 |
| LP-PLA2 (ng/ml) | 152,0± 48,6 | 217,7± 100,5 | 0.005 |
| hs-CRP (mg/dl) | 3,1±3,8 | 3,2±2,7 | 0.90 |
| TAC (mM) | 1,36±0,53 | 1,03±0,51 | 0.05 |
| Fasting blood glucose (mg/dl) | 81,3±21,8 | 82,7±16,3 | 0.79 |
| Total cholesterol (mg/dl) | 184,0± 35,7 | 213,5±43,0 | 0,01 |
| Triglyceride (mg/dl) | 167,0 ±76,2 | 247,9±175,3 | 0.05 |
| Creatinine (mg/dl) | 0,92±0,12 | 0,92±0,11 | 0.83 |
| AHI | 35,4±21,7 | 29,1±25,1 | 0.35 |
| Total sleep time (minute) | 351,0± 62,7 | 330,1± 72,9 | 0.26 |
| Lowest sat O2 (%) | 80,1±5,8 | 81,7±4,7 | 0.58 |
hs-CRP: high sensitive C-reactive protein, BMI: Body mass index, AHI: apnea-hipopne index, TAC: Total antioxidant capacity. *Hypertension was defined as resting blood pressure ≥140/90 mmHg
Stepwise Linear regression analysis to determine of independent predictors of the arousal index
| B | Std. Error | Beta | t | P | |
|---|---|---|---|---|---|
| Constant | 11.011 | 31.577 | 0.349 | 0.73 | |
| Age | 0.246 | 0.209 | 0.165 | 1.175 | 0.25 |
| Systolic BP (mmHg) | -0.092 | 0.197 | -0.064 | -0.464 | 0.65 |
| Apnea-hipopne index | 0.151 | 0.092 | 0.231 | 1.638 | 0.11 |
| Total cholesterol (mg/dl) | 0.036 | 0.075 | 0.099 | 0.488 | 0.63 |
| Triglyceride (mg/dl) | 0.021 | 0.020 | 0.194 | 1.057 | 0.30 |
| hs-CRP | -0.038 | 0.664 | -0.008 | -0.057 | 0.96 |
| LP PLA2 (ngml) | 0.063 | 0.028 | 0.357 | 2.226 | 0.03 |
| TAC (mM) | -3.684 | 3.629 | -0.141 | -1.015 | 0.32 |
| BMI | -0.530 | 0.566 | -0.156 | -0.936 | 0.36 |
| Dependent Variable:, R=0,59, R2=0,35, F(9,46)=2.20, P=0,04 (The first step of the stepwise linear regression model) | |||||
| Constant | 3.793 | 4.895 | 0.775 | 0.443 | |
| LP PLA2 (ngml) | 0.077 | 0.024 | 0.437 | 3.259 | 0.002 |
| R= 0.44, R2=0.21, F(1,46) =10.62, P=0.002 (the last step of stepwise linear regression model) | |||||
hs-CRP: high sensitive C-reactive protein, BMI: Body mass index. BP: Blood pressure, TAC: Total antioxidant capacity
Correlation analysis between total arousal and laboratory tests, demographic characteristics
| r | p | |
|---|---|---|
| Age | 0.12 | 0.38 |
| BMI | -0.20 | 0.19 |
| Hip/waist circumference ratio | 0.08 | 0.57 |
| Systolic Blood pressure | 0.07 | 0.65 |
| Diastolic blood pressure | 0.10 | 0.47 |
| LP-PLA2 (ng/ml) | 0.43 | 0.002 |
| TAC (mM) | -0.29 | 0.04 |
| hs-CRP | 0.06 | 0.66 |
| Fasting blood glucose | -0.10 | 0.50 |
| Creatinin | -0.09 | 0.51 |
hs-CRP: high sensitive C-reactive protein, BMI: Body mass index, TAC: total antioxidant capacity
Correlation analysis between LP-PLA2 and polysomnography parameters, other laboratory findings
| r | p | |
|---|---|---|
| Total arousal | 0.43 | 0.002 |
| Apnea index | 0.03 | 0.832 |
| Apnea-hypopnea index | -0.08 | 0.573 |
| Total sleep time | -0.27 | 0.06 |
| PLM index | 0.49 | 0.001 |
| Lowest saturation (%) | 0.11 | 0.69 |
| Age (years) | 0.07 | 0.65 |
| BMI | -0.35 | 0.01 |
| Waist / Hip circumference ratio | -0.23 | 0.12 |
| Systolic Blood pressure (mmHg) | 0.20 | 0.17 |
| Diastolic blood pressure (mmHg) | 0.09 | 0.53 |
| hs-CRP (mg/dl) | 0.27 | 0.06 |
| TAC (mM) | -0.17 | 0.24 |
| Fasting blood glucose (mg/dl) | -0.07 | 0.65 |
| Total cholesterol (mg/dl) | 0.09 | 0.54 |
| Triglyceride (mg/dl) | 0.02 | 0.91 |
| Creatinine (mg/dl) | -0.19 | 0.21 |
hs-CRP: high sensitive C-reactive protein, BMI: Body mass index, PLM: Periodic Limb Movement
Discussion
This study first demonstrated that arousal index was positively correlated with LP-PLA2 levels and was negatively correlated with total antioxidant capacity. The arousal index is an important index of sleep fragmentation and the restorative quality of sleep. These results may explain the increased cardiovascular risk during arousal.
Large randomized controlled trials identified higher circulating Lp-PLA2 levels as an independent predictor for first-time acute myocardial infarction (AMI), cardiac death and stroke [15-18]. The Rotterdam study demonstrated a higher risk of AMI and stroke especially in patients with Lp-PLA2 activity above the lowest quartile [19]. According to the knowledge, circulating Lp-PLA2 levels were not previously studied in patients with OSA. Due to the circulating Lp-PLA2 levels, patients in the highest arousal quartile may have been increased risk of coronary and/or cerebral event compared to the patients with lowest and second arousal quartiles. Also, the linear association between arousal index and LP-PLA2 was independent from baseline demographic characteristics. But this relation was not valid to the total cholesterol and triglyceride levels. So, Lp-PLA2 levels may be better marker of cardiovascular risk in OSA especially with higher arousal index.
Arousals are associated with acute surges in blood pressure and heart rate [5-7]. Cardiovascular activity is closely modulated during respiratory events; arousals show a particularly powerful effect on sympathetic activity in OSA patients [7, 9]. In patients with OSA, the cardiovascular response to a postapneic arousal is two fold than to a spontaneous arousal [25]. Arousal-induced tachycardia, which generally corresponds to periods of high cardiac pre- and after-load and reduced myocardial oxygen delivery at the termination of respiratory events, may provide a hemodynamic disturbance substrate for initiating cardiac event and potentially sudden cardiac death. The elevated circulating LP-PLA2 levels in patients with higher arousal may contribute to this hypothesis.
Although the reasons for prevalent nocturnal death in OSA remain unclear, three reasonable causes are commonly accused by the investigators. These factors are cardiac arrhythmias, stroke and myocardial infarction. Firstly, the potential mechanisms of sudden cardiovascular death in OSA patients have been based on the cardiac electrical disturbances [10]. The QT interval, reflecting the overall duration of ventricular repolarization, has been shown to be prolonged during apnea and suddenly shortened during the hyperventilation phase post apnea [11]. In healthy volunteers, arousals from non-rapid eye movement (NREM) sleep consistently produced QT interval shortening and PR interval prolongation [12], a response potentially prone to re-entry phenomena and consequently for arrhythmogenesis [27]. Secondly, it has been attributed to cardiovascular mechanical stressors such as frequent apneas causing increases in negative intrathoracic pressure and cardiac pre-and after-load work; and intermittent hypoxemia via causing endothelial dysfunction [28]; and haemostatic disturbances [29-31]. Finally, the present study demonstrated that higher arousal index was associated with increased LP-PLA2 level. The elevated levels of circulating LP-PLA2 may explain prone to sudden death due stroke and AMI in patients with OSA and increased arousal index.
Total antioxidant capacity
Jelic et al. demonstrated that NO availability and circulating endothelial progenitor cell levels, a marker of endothelial repair capacity were decreased in patients with OSA compared to control subjects [22]. In addition, continuous positive airway pressure (CPAP) therapy improved these parameters [22]. The present study was found that there was significantly negative correlation between total antioxidant capacity and arousal. Also, it was demonstrated that total antioxidant capacity was significantly decreased in patients above median arousal. To knowledge, the relationship between arousal and total antioxidant capacity has not been mentioned in current literature. LP-PLA2 level was related with vulnerable plaque and decreasing antioxidant capacity may be additive effect with LP-PLA2 on cholesterol plaque. Thus, the plaque vulnerability to rupture has increases.
hs-CRP is a well-known conventional cardiovascular risk marker and it was not related with arousal index. This is the major negative result of the study. A possible explanation of this negative result that sensitivity of hs-CRP may be decrease due to the lower conventional risk factors of study subjects such as the lower percentage of patients with hypertension, diabetes mellitus, smoking and hypercholesterolemia. In addition, small number of the patients may be the cause of the limited difference between groups.
Limitation
The major limitation of the study is small number of the patients and absence of a control group without OSA and this was because of limited financial support. However this study may be accepted as a pilot study. Another issue is the exclusion of the women from the study. Hormone replacement therapy at the postmenopausal period affects the relation between LP-PLA2 and vascular events [32, 33]. In addition, the effect of menopause on LP-PLA2 levels is unclear. Based on the current literature, value of Lp-PLA2 in women is controversial.
Conclusion
Despite these limitations, this study demonstrated that arousal was not only associated with increased sympathetic nervous system activation and electrocardiographic changes but also these patients were prone to cardiovascular and cerebrovascular events via elevated levels of LP-PLA2, which is a marker of increased plaque vulnerability. In addition, total antioxidant capacity, a marker of body defense system against increased oxidative stress was decreased. These results need a confirmation with a large prospective follow up study.
Acknowledgements
The authors acknowledge to Bilim Drug Company Ltd. for the financial support for the LP-PLA2 and total antioxidant capacity assay kits. Also, the authors acknowledge to sleep laboratory staff for their contributions to the study.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Peppard PE, Young T, Palta M. et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384
2. Varol E, Akcay S, Ozaydin M. et al. Influence of obstructive sleep apnea on left ventricular mass and global function: sleep apnea andmyocardialperformanceindex. Heart And Vessels. 2010;25(5):400-404
3. Abe H, Takahashi M, Yaegashi H. et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart And Vessels. 2010;25(1):63-69
4. Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol. 1995;79:151-162
5. Davies RJ, Belt PJ, Roberts SJ, Ali NJ. Stradling JR Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123-1130
6. Pitson D, Chhina N, Knijn S, van Herwaaden M, Stradling J. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects. Clin Sci. 1994;87:269-273
7. Trinder J, Padula M, Berlowitz D. et al. Cardiac and respiratory activity at arousal from sleep under controlled ventilation conditions. J Appl Physiol. 2001;90:1455-1463
8. Nieto FJ, Young TB, Lind BK. et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829-1836
9. O'Driscoll DM, Meadows GE, Corfield DR, Simonds AK, Morrell MJ. Cardiovascular response to arousal from sleep under controlled conditions of central and peripheral chemoreceptor stimulation in humans. J Appl Physiol. 2004;96:865-870
10. Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490-494
11. Gillis AM, Stoohs R, Guilleminault C. Changes in the QT interval during obstructive sleep apnea. Sleep. 1991;14:346-350
12. Nalivaiko E, Catcheside PG, Adams A, Jordan AS, Eckert DJ, McEvoy RD. Cardiac changes during arousals from non- REM sleep in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1320-1327
13. Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med. 2000;45:391-418
14. Blann AD, Seigneur M, Steiner M, Miller JP, McCollum CN. Circulating ICAM-1 and VCAM-1 in peripheral artery disease and hypercholesterolaemia: relationship to the location of atherosclerotic disease, smoking, and in the prediction of adverse events. Thromb Haemost. 1998;79:1080-1085
15. Packard CJ, O'Reilly DS, Caslake MJ. et al. Lipoproteinassociated phospholipase A2, as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148-1155
16. Ballantyne CM, Hoogeveen RC, Bang H. et al. Lipoprotein-associated phospholipase A2, high sensitive c-reactive protein and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837-842
17. Ballantyne CM, Hoogeveen RC, Bang H. et al. Boerwinkle E. Lipoprotein-associated phospholipase A2, high sensitive C-reactive protein and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med. 2005;165:2479-2484
18. Koenig W, Khuseyinova N, Löwel H, Trischler G, Meisinger C. Lipoproteinassociated phospholipase A2 adds to risk prediction of incident coronary events by Creactive protein in apparently healthy middle-aged men from the general population. Results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903-1908
19. Oei HH, van der Meer IM, Hofman A. et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam study. Circulation. 2005;111:570-575
20. Brilakis ES, McConnell JP, Lennon RJ, Elesber AA, Meyer JG, Berger PB. Association of lipoprotein-associated phospholipase A2 levels with coronary artery disease risk factors, angiographic coronary artery disease, and major adverse events at follow-up. Eur Heart J. 2005;26:137-144
21. Koenig W, Twardella D, Brenner H, Rothenbacher D. Lipoproteinassociated phospholipase A2 predicts future cardiovascular events in patients with coronary heart disease independently of traditional risk factors, markers of inflammation, renal function, and hemodynamic stress. Arterioscler Thromb Vasc Biol. 2006;26:1586-1593
22. Jelic S, Padeletti M, Kawut SM. et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270-2278
23. Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, Ill: American Academy of Sleep Medicine. 2007
24. Davidson MH, Corson MA, Alberts MJ. et al. Consensus Panel Recommendation for Incorporating Lipoprotein-Associated Phospholipase A2 Testing into Cardiovascular Disease Risk Assessment Guidelines. Am J Cardiol. 2008;101:51F-57F
25. Okabe S, Hida W, Kikuchi Y. et al. Role of hypoxia on increased blood pressure in patients with obstructive sleep apnoea. Thorax. 1995;50:28-34
26. Trinder J, Ivens C, Kleiman J, Kleverlaan D, White DP. The cardiorespiratory activation response at an arousal from sleep is independent of the level of CO(2). J Sleep Res. 2006;15:174-182
27. Maison-Blanche P, Coumel P. Changes in repolarization dynamicity and the assessment of the arrhythmic risk. Pacing Clin Electrophysiol. 1997;20:2614-2624
28. Dincer HE, O'Neill W. Deleterious effects of sleep-disordered breathing on the heart and vascular system. Respiration. 2006;73:124-130
29. Steiner S, Jax T, Evers S. et al. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology. 2005;104:92-96
30. Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: data from randomised controlled trials. Thorax. 2004;59:777-782
31. Geiser T, Buck F, Meyer BJ, Bassetti C, Haeberli A, Gugger M. In vivo platelet activation is increased during sleep in patients with obstructive sleep apnea syndrome. Respiration. 2002;69:229-234
32. Wassertheil-Smoller S, Kooperberg C, McGinn AP. et al. Lipoprotein-associated phospholipase A2, hormone use, and the risk of ischemic stroke in postmenopausal women. Hypertension. 2008;51(4):1115-22
33. Brilakis ES, Khera A, McGuire DK. et al. Influence of race and sex on lipoprotein-associated phospholipase A2 levels: observations from the Dallas Heart Study. Atherosclerosis. 2008;199(1):110-5
Author contact
![]() Corresponding author: Taha Tahir Bekci, MD. Konya Education and Research Hospital, Meram 42090 Konya/Turkey. Phone: +90 533 3787676. E-mail: tahabekcicom
Corresponding author: Taha Tahir Bekci, MD. Konya Education and Research Hospital, Meram 42090 Konya/Turkey. Phone: +90 533 3787676. E-mail: tahabekcicom

 Global reach, higher impact
Global reach, higher impact