3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(3):222-230. doi:10.7150/ijms.8.222 This issue Cite
Research Paper
Characterization of Human Erythrocytes as Potential Carrier for Pravastatin: An In Vitro Study
Department of Pharmaceutics, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh 11451, Saudi Arabia
Received 2011-1-4; Accepted 2011-2-18; Published 2011-3-11
Abstract
Drug delivery systems including chemical, physical and biological agents that enhance the bioavailability, improve pharmacokinetics and reduce toxicities of the drugs. Carrier erythrocytes are one of the most promising biological drug delivery systems investigated in recent decades. The bioavailability of statin drugs is low due the effects of P-glycoprotein in the gastro-intestinal tract as well as the first-pass metabolism. Therefore in this work we study the effect of time, temperature as well as concentration on the loading of pravastatin in human erythrocytes to be using them as systemic sustained release delivery system for this drug. After the loading process is performed the carriers' erythrocytes were physically and cellulary characterized. Also, the in vitro release of pravastatin from carrier erythrocytes was studied over time interval. Our results revealed that, human erythrocytes have been successfully loaded with pravastatin using endocytosis method either at 25oC or at 37oC. The loaded amount at 10 mg/ml is 0.32mg/0.1 ml and 0.69 mg/0.1 ml. Entrapment efficiency is 34% and 94% at 25oC and 37oC respectively at drug concentration 4 mg/ml. Moreover the percent of cells recovery is 87-93%. Hematological parameters and osmotic fragility behavior of pravastatin loaded erythrocytes were similar that of native erythrocytes. Scanning electron microscopy demonstrated that the pravastatin loaded cells has no change in the morphology. Pravastatin releasing from carrier cell was 83% after 23 hours in phosphate buffer saline and decreased to 72% by treatment of carrier cells with glutaraldehyde. The releasing pattern of the drug from loaded erythrocytes obeyed first order kinetics. It concluded that pravastatin is successfully entrapped into erythrocytes with acceptable loading parameters and moderate morphological changes, this suggesting that erythrocytes can be used as prolonged release for pravastatin.
Keywords: drug delivery, erythrocytes, pravastatin, osmotic fragility
Introduction
The statin drugs are used in the treatment of hypercholesterolemia; moreover, these drugs have pleiotropic effect, so that they are used in the treatment of many diseases such as osteoporosis, Alzheimer disease, organ transplantation, stroke and diabetes [1]. Administration of statins by oral rout is associated with several problems including diarrhea, constipation, indigestion and nausea [2]. Also the bioavailability of these drugs is low due the effects of cytochrome and P-glycoprotein (Pgp) in the gastro-intestinal tract as well as the first-pass metabolism in the liver [3]. Therefore, the increased dosage of statin drugs is usually used to obtain the desired therapeutic efficacy but increasing the dose of these drugs may exaggerate the side effects on the liver, kidney and muscular tissue [3].
The pharmacologically active form of pravastatin is open hydroxyl-acid so that its hydrophilicity is markedly higher than that of other statins. The oral bioavailability of this statin is low due to degradation in the stomach and incomplete absorption [4]. Therefore, several strategies are used for improvement both pharmacokinetics and pharmacodynamic properties of statins including inhibition of the metabolism [5], administration of statins with certain juices [6] or inhibition of Pgp [3].
Unfortunately these strategies are frequently associated with increase the risk of side effects of the statins [3,7].Therefore the developments of novel pharmaceutical formulations are used as alternative approaches to improve the bioavailability and therapeutic efficacy these drugs [8-10]. Several studies have been suggested different pharmaceutical devices like nanoparticles, microparticles [11], and drug-loaded erythrocytes [12, 13].
Carrier erythrocytes are one of the biological drug delivery systems investigated in recent decades. They are biologically compatible and have large volumes; therefore, they are well suited to be used as drug carriers. Additionally, they can be used as substitute biological carriers such as liposomes or nanoparticles that have been used for the encapsulation of therapeutic agents [14]. According to the desired therapeutic strategy erythrocytes are used either as a carrier for sustained release of the drugs or as carriers to deliver and target drugs to specific organs [15].
Therapeutic agents can be loaded in erythrocytes either by physical methods such as endocytosis and osmosis-based systems or by chemical perturbation of the erythrocytes membrane [16]. Endocytosis is the process by which cells absorb molecules by engulfing them. It is used by all cells of the body because most substances important to them are large polar molecules that cannot pass through the hydrophobic plasma or cell membrane [17]. Drug loading into erythrocyte by endocytosis is more preferable when they used sustained released carriers, because it has minimal effects on erythrocytes structure and morphology. The substances to be entrapped into the erythrocytes should have a degree of water solubility and resistance to degradation within erythrocytes [18]. Certain drugs have been entrapped in erythrocytes by endocytosis, including vinblastine, chlorpromazine, hydrocortisone, propranolol, tetracaine, retinol, and primaquine [16, 19].
The current work aims to study the encapsulation of pravastatin in human carrier erythrocytes by endocytosis method. The entrapment efficiency of the drug at different times, temperatures as well as different initial concentrations of this statin was investigated. The hematologic parameters and osmotic fragility of the loaded carrier erythrocytes were evaluated. Additionally, the in vitro release of pravastatin from carrier erythrocytes was measured over time.
Materials and methods
Materials
The chemicals used in this study were pravastatin sodium(SPIMACO, Riyadh, Saudi Arabia), NaCl (Merck, Germany), KCl (Fluka chemie AGCH), Na2HPO4·12H20 (BDH-GPRTm), KH2PO4 (Merck, Germany), MgCl2·6H2O (Avonchem Limited), MgSO4.7H2O (Sigma Chemical Co., St. Louis, Mo), Glucose (Panreac), NaHCO3 (Panreac), adenosine 5-triphosphate (Spectrum chemical MFG. CORP) glutaraldehyde and acetonitrile (HPLC grade) and methanol from acquired from (BDH). All remaining chemicals were of analytical grade.
Instrumentation
A Coulter® AC.T diffTM hematology analyzer (Beckman Coulter, Inc., Brea, CA, USA); a Spectro UV-Vis Split Beam PC, model UVS-2800 (Labomed, Inc., Culver City, CA, USA); Chromatography was performed by reversed phase ultra performance liquid chromatography (UPLC). Acquity® (UPLC) system, using Acquity® UPLC BEH C18 column (1.7 μm, 2.1 mm x 50 mm) obtained from Waters (Waters Inc., Bedford, MA, USA). Water bath (Julabo SW22), Jencons 375H sonicator, Hettic EBA 20 and Hettic MIKRO 20(Germany) centrifuges were used in these investigations.
Preparation of erythrocyte suspension
The blood specimens were collected from apparently healthy donors not suffered from acute and chronic diseases. Informed consent was obtained from each of the donors. Blood samples were collected in heparinized vacutainers and centrifuged for 5 min at 5000 rpm. The plasma and the buffy coat were removed by aspiration. Erythrocytes were washed three times in cold phosphate buffer saline (PBS) with centrifugation for 5 min at 5000 rpm [20, 21]. The experimental protocol was approved by the research center ethics committee of King Saud University College of Pharmacy, Riyadh, Saudi Arabia.
Pravastatin loading procedures
The hematocrite of washed erythrocytes was adjusted by PBS to 45%. In 2 ml eppendorff tubes, 400 µl of suspension are added to 400 µl of PBS containing the known concentration of the drug and 2.5 mmol of ATP, 2.5 mmol MgCl2 and 2.5 mmol of CaCl2, gently mixing to avoid hemolysis and incubation for 15 minutes at room temperature. The erythrocytes suspension is centrifuged for 5 min at 5000 rpm and the supernatant is discarded. The packed erythrocytes was washed 2 times in cold BPS with centrifugation for 5 min at 5000 rpm [22].
Study the effect of concentration
To determine the effect of drug concentration on loading efficiency we use different drug concentrations (2 mg, 4 mg, 8 mg, and 10 mg) for all selected incubation times, and compare results to obtain the more suitable concentration for loading process which produce most excellent loading parameters [23].
Study the effect of time
The effect of time on loading efficiency and loading process was done for the previous concentrations for different times (15, 30, 60, 120 minutes) and compare the results [24].
Study the effect of temperature
The loading process was done at 25oC and 37oC for the previous different times and concentrations.
Loading parameters
To evaluate the final erythrocyte carriers, three indices were defined as loading parameters (loaded amount, entrapment efficiency and cell recovery) [25].
Loaded amount
The total amount of pravastatin entrapped in 0.1 ml of the final packed erythrocytes.
Efficiency of entrapment
The percentage of the loaded amount of pravastatin to the total amount of that added during the entire loading process.
Cell recovery
The percentage ratio of the hematocrite value of the final loaded cells to that of the initial packed cells, both measured using equal suspension volumes.
In vitro characterization of pravastatin loaded erythrocytes
Hematological Indices
To determine the effect of loading process on erythrocytes, normal erythrocytes, erythrocytes suspended in PBS, and pravastatin-loaded erythrocytes were counted. The mean corpuscular volume (MCV: mean cell volume), the mean corpuscular hemoglobin (MCH: average hemoglobin content per each cell), and the mean corpuscular hemoglobin content (MCHC: hemoglobin content per 100 ml of cell volume) were measured using Coulter® LH 780 hematology analyzer [24].
Determination of osmotic fragility behavior of loaded erythrocytes
Erythrocytes resistance against lysis as a result of the osmotic pressure changes of their surrounding media was evaluated. Twenty five μl of erythrocyte sample was added to each of a series of 2.5 ml saline solutions containing 0.0 to 0.8 g% of NaCl. After gentle mixing and standing for 15 min at room temperature, the erythrocyte suspensions were centrifuged at 5000 rpm for 5 min. The absorbance of the supernatant was measured at 540 nm [26]. The absorbance percentage released hemoglobin was expressed as percentage absorbance of each sample in correlation to a completely lysed sample prepared by diluting of packed cells of each type with 1.5 ml of distilled water. Osmotic fragility was studied for each drug concentration.
In vitro releasing study
The release of pravastatin as well as hemoglobin from carrier erythrocytes were determined as following, 1 ml of packed drug-loaded erythrocytes was diluted to 10 ml using PBS the suspension was mixed thoroughly by several gentle inversions. Then, the mixture was divided into ten 0.5 ml portions in 1.5 ml eppendorf tube. The samples were rotated vertically while incubated at 37◦C. At the beginning of the test and also at 0.25, 0.5, 1, 2, 8, 20, and 23 h intervals, one of the samples was harvested and then centrifuged at 3000 for 5 min. One hundred μl of the supernatants were separated for drug assay. In addition, the absorbance of a 0.3 ml portion of the supernatant was determined at 540 nm using a spectrophotometer. Hemoglobin release were determined in reference to a completely lysed sample[15]. The release of drug was studied also in plasma and in PBS after addition of glutaraldehyde.
Pravastatin assay by UPLC
A reversed phase UPLC method was developed and used throughout the study for pravastatin assay. The mobile phase in this method consists of acetonitrile and water with ratio 35:65, the flow rate was 0.5 ml/min. The analyte separation was carried out using C18 column under temperature 40º C using UV detector at 237 nm.
To determine the amount of loaded pravastatin, the erythrocyte pellets were hemolysed by addition of equal volume of distilled water with strong shaking to ensure erythrocyte hemolysis. The proteins were precipitated by addition of 1ml methanol, mixed well and vortexed for 15 minute and then centrifuged at 13000 rpm for 15 minutes. The supernatant is taken and filtered using 0.22 Millipore disposable filters and then complete the volume to 5ml by water. 1µl of filtrate was injected to the UPLC.
Scanning electron microscopy (SEM)
A JEOL JSM-6380 LA scanning electron microscope (Jeol Ltd., Tokyo, Japan) equipped with a digital camera, at 20 kV accelerating voltage was used to evaluate the morphological differences between normal and pravastatin loaded erythrocytes. Both normal and 8 mg/ml pravastatin -loaded erythrocyte samples were processed as follows. After the samples were fixed in buffered glutaraldehyde, the aldehyde medium was drained off. The cells were rinsed 3 times for 5 min in phosphate buffer and post-fixed in osmium tetroxide for 1 h. The samples were then rinsed with distilled water and dehydrated using a graded ethanol series: 25, 50, 75, 100, and another 100%, each for 10 min. The samples were rinsed in water, removed, mounted on studs, sputter-coated with gold, and then viewed using SEM [25].
Statistical analysis
The statistical differences between native and loaded erythrocytes were analyzed by one way ANOVA followed by the Bonferroni multiple comparison test, using PASW Statistics 18 Software, v. 5.01 (SPSS Software, Inc.). The results with p<0.01 were considered statistically significant.
Results and discussion
Analysis method validation
The new invented method of pravastatin sodium extraction and assay in erythrocytes using UPLC was validated according to FDA guidelines. Recovery of extraction method was (96%-108%), the analysis method was selective to the drug with accuracy (98%-103%) and precision (0.3-6.4). All the tested parameters were in acceptable levels.
Encapsulation of pravastatin in human erythrocytes
The current work studies effect of time, temperature as well as drug concentration on the process of pravastatin loading into human erythrocytes by endocytosis method as trial to obtain pravastatin prolonged release system. The results show that the highest level of pravastatin loaded on erythrocytes was attained using 10 mg/ml of the drug, at 37oC and 2 hours incubation time. While at 25oC the maximum drug loading is attained after 1hour.
This result in agreement with previous study demonstrated the increase in the cell membrane activity upon temperature increase till reach optimum temperature 37oC [27]. Also this find is supported by another study shows that, endocytosis process is decreased by decreasing temperature[17], Therefore drug loading at 37oC is greater than at 25oC. Table 1 display the effect of pravastatin concentration and incubation time on the amount of pravastatin loaded on human carrier erythrocytes at 25oC while table 2 display the same parameters at 37oC.
Effect of pravastatin concentration and incubation time on the amount of pravastatin loaded on human carrier erythrocytes at 25oC by endocytosis
| Drug concentration(µg/ml) | Drug incubation times | |||
|---|---|---|---|---|
| 15 min. | 30 min. | 60 min. | 120 min. | |
| 2× 103 | 292 ± 15.5 | 395 ± 40.1* | 532 ± 39.1* | 544 ± 23.0 |
| 4× 103 | 415 ± 20.0# | 971 ± 3 7.1*# | 1366 ± 63.5*# | 1286 ± 36.5# |
| 8× 103 | 627 ± 26.8# | 2006 ± 36.6*# | 2561 ± 111*# | 2499 ± 46.0# |
| 10× 103 | 706 ± 28.5# | 2352 ± 42.0*# | 3211 ± 66.5*# | 3170 ± 134# |
Data were tested by one-way analysis of variance and represented as mean ± SD. Six samples in each group (N = 6). Bonferroni multiple comparison tests using SPSS software was performed to determine differences between mean values. * Significantly different according to time at p < 0.01, # significantly different according to concentration at p < 0.01.
Effect of pravastatin concentration and incubation time on the amount of pravastatin loaded on human carrier erythrocytes at 37oC by endocytosis
| Drug concentration(µg/ml) | Drug incubation times | |||
|---|---|---|---|---|
| 15 min. | 30 min. | 60 min. | 120 min. | |
| 2× 103 | 223 ± 23.5 | 1047 ± 127* | 1317 ± 70.0 | 1663 ± 245 |
| 4× 103 | 489 ± 19.0# | 1795 ± 13.7*# | 3031 ± 297*# | 3788 ± 339*# |
| 8× 103 | 907 ± 14.5# | 2074 ± 267* | 4169 ± 222*# | 5540 ± 479*# |
| 10× 103 | 1047 ± 33.5# | 2725 ± 241*# | 4690 ± 178*# | 6900 ± 88.0*# |
Data were tested by one-way analysis of variance and represented as mean ± SD. Six samples in each group (N = 6). Bonferroni multiple comparison tests using SPSS software was performed to determine differences between mean values. * Significantly different according to time at p < 0.01, # significantly different according to concentration at p < 0.01
The presence of agents like tonicity as well as an energy source stimulate the endocytosis [28]. In our demonstration, the presence of calcium ions as well as ATP stimulates the endocytosis of pravastatin by erythrocytes. This is supported by the observation of Schrier et al., which reported that the calcium ions and energy source stimulate drug uptake by erythrocytes through membrane invagination and formation of endocytotic vacuoles [29]. The drugs induced endocytosis is dependent on the persistence of erythrocyte energy sources [30].
Also, the results of this demonstrated that the loading of pravastatin into erythrocytes was directly proportional with increase of drug concentration in the incubation medium, in the 2-10 mg/ml concentration range. This finding is in concurrence with results reported by Millan [24], and Hamidi [25, 31].
Loading parameters
Loaded amount
The loaded amounts of pravastatin at 25ºC and 37ºC were determined; at 25 ºC the highest loaded amount was 0.32 mg while it is 0.69 mg at 37 ºC. These loaded amounts are suitable to pravastatin dosing upon reinjection of low volumes of drug loaded erythrocytes to the host body. This demonstration was stated in another studies by Bossa [32] and Hamidi [15, 25], according to similar results.
Loading efficiency at 25oC
The effect of concentration and incubation time on the percent of drug loading is shown in figures 1 and 2 at 25ºC and at 37ºC. The percent is of drug loading was started from 15% after 15 minutes upon using drug concentration 2 mg at 25oC, and decreased upon increasing concentration. The higher percent was 34% that given at 4 mg after 60 minutes.
Loading efficiency at 37ºC
The results obtained at 37oC were much better than the one obtained at 25oC. The loading efficiency reaches 94% at 4mg after 120 minutes, while decrease upon increasing concentration. This loading efficiency is better than that obtained in primaquine loading study as comparison [33].
Cell recovery
A cell recovery of loading process was 87-93%, this is practically better than the recovery results of other studies such as primaquine and enalaprilat [33, 34]. This result is may be an evident to the quite effect of loading process on erythrocytes and/or protective effect of pravastatin as investigated in previous study stated that the pravastatin protect erythrocytes against oxidative damage induced by drugs [35].
Effect of pravastatin incubation time and drug concentration on the percent of pravastatin loading on human carrier erythrocytes at 25oC by endocytosis. The highest loading efficiency obtained when concentration 4 mg/ml is used for incubation time 1 hour. Data is expressed as mean ± SD, Six samples in each group (N = 6).
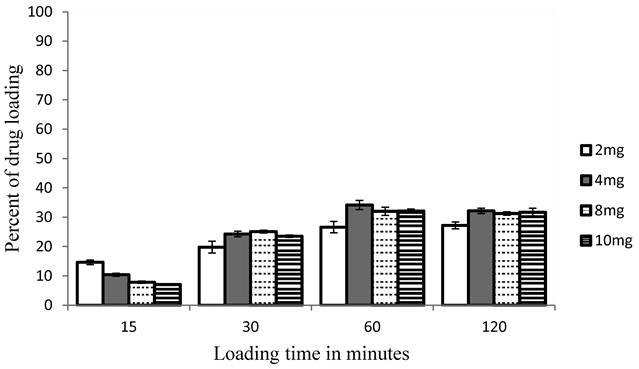
Effect of pravastatin incubation time and drug concentration on the percent of pravastatin loading on human carrier erythrocytes at 37oC by endocytosis. The highest loading efficiency obtained when concentration 4mg/ml is used for incubation time 2 hours. Data is expressed as mean ± SD, Six samples in each group (N = 6).

Hematological parameters of control erythrocytes and loaded erythrocytes obtained with different concentrations of pravastatin (mg/ml)
| Control | Sham encapsulated | 2 mg/ml | 4 mg/ml | 8mg/ml | 10 mg/ml | |
|---|---|---|---|---|---|---|
| Hct (%) | 51.6 ± 2.04 | 44.9 ± 0.21 | 45.9 ± 1.49 | 45.3 ± 3.68 | 48.0 ± 2.20 | 51.6 ± 2.04 |
| MCV (fl) | 84.1 ± 0.32 | 82.3 ± 0.57 | 83.6 ± 0.47 | 82.9 ± 1.25 | 83.4 ± 0.46 | 81.2 ± 0.36* |
| MCH (pg) | 28.2 ± 0.68 | 28.2 ± 0.03 | 27.6 ± 0.20 | 27.7 ± 0.06 | 27.8 ± 0.15 | 27.3 ± 0.10** |
| MCHCH (gm/dl) | 33.5 ± .035 | 34.2 ± 0.28 | 33.0 ± 0.46 | 33.4 ± 0.38 | 33.4 ± 0.29 | 33.6 ± 0.27 |
Data were tested by one-way analysis of variance and represented as mean ± SD. Three samples in each group (N = 3). Bonferroni multiple comparison tests using SPSS software was performed to determine differences between mean values at (P ≤ 0.01).* significantly different from the control at p < 0.01, ** significantly different from the control at (p< 0.05). Mean corpuscular volume (MCV), Mean corpuscular hemoglobin (MCH), Mean corpuscular hemoglobin concentration (MCHC).
In vitro characterization of pravastatin loaded erythrocytes
Hematological Indices
The hematological parameters, such as MCV, MCH and MCHC were characterized. These parameters determine the influence of the encapsulation process on the hematological properties of the erythrocytes [24]. Table 3 represent the mean hematological parameters of the pravastatin loaded erythrocytes obtained with different pravastatin concentrations and values for the same cells before the loading procedures (the control cells) and after loading process but without using drug(sham encapsulated).
The results show non-significant change in hematological parameters is observed except MCV at higher concentrations (10 mg). From these data pravastatin loading into erythrocytes occurs either by encapsulation or binding to the cell membrane[36] and also the loading procedure does not affect the MCV. This finding is in agreement with previous report[34]. Non-significant change in both MCH and MCHC can be explained by pravastatin preserve a physical and/or functional barrier of erythrocyte, therefore prevent hemoglobin loss from carrier erythrocytes. These predictions are supported the SEM analysis data and osmotic fragility that will discussed later.
Osmotic fragility behavior of pravastatin loaded erythrocytes
Osmotic fragility determines the susceptibility of erythrocytes to osmotic lysis in respect to serial dilution of NaCl. The data obtained shows that there is no significant difference in the osmotic fragility of loaded erythrocyte at 2, 4, 8 mg/ml pravastatin when compared to that of unloaded erythrocytes (Table 4).
Erythrocyte osmotic fragility of unloaded erythrocytes and erythrocytes loaded with 8, 4 and 2 mg/ml pravastatin. Values are percent hemolysis in corresponding salt concentrations
| NaCl % | Control | 2 mg | 4 mg | 8 mg |
|---|---|---|---|---|
| 0.80 | 1.30 ± 0.66 | 0.54 ± 0.01 | 0.92 ± 0.01 | 1.19 ± 0.01 |
| 0.70 | 5.35 ± 1.34 | 0.74 ± 0.01 | 1.23 ± 0.01 | 2.80 ± 0.02 |
| 0.60 | 12.3 ± 1.97 | 7.26 ± 0.07 | 9.53 ± 0.06 | 8.99 ± 0.07 |
| 0.50 | 29.7 ± 3.42 | 15.0 ± 0.03 | 17.2 ± 0.04 | 15.2 ± 0.04 |
| 0.45 | 56.5 ± 3.50 | 55.6 ± 0.08 | 71.2 ± 0.09 | 73.2 ± 0.10 |
| 0.40 | 74.1 ± 3.04 | 73.5 ± 0.12 | 88.7 ± 0.14 | 94.0 ± 0.17 |
| 0.35 | 80.45 ±1.56 | 76.8 ± 0.11 | 93.9 ± 0.09 | 94.1 ± 0.16 |
| 0.30 | 84.4 ± 4.14 | 77.4 ± 0.11 | 95.8 ± 0.12 | 97.7 ± 0.12 |
| 0.20 | 89.6 ± 4.60 | 78.6 ± 0.12 | 97.7 ± 0.10 | 99.0 ± 0.02 |
| 0.10 | 96.8 ± 4.80 | 80.8 ± 0.5 | 100 ± 0.11 | 100 ± 0.03 |
| 0.00 | 100 ±0.05 | 100 ± 0.01 | 100 ± 0.11 | 100 ± 0.03 |
Data were tested by one-way analysis of variance and represented as mean ± SD. Three samples in each group (N = 3). Bonferroni multiple comparison tests using SPSS software was performed to determine differences between mean values at (P ≤ 0.01).
Several studies reported the effect of drugs on fragility behavior of erythrocytes. Hamidi et al., stated that osmotic fragility of loaded erythrocytes is lower than unloaded cells [34]. On contrast, another study revealed that osmotic fragility of carrier erythrocytes is higher than unloaded cells [37]. In The present study, the behavior of pravastatin loaded erythrocytes towards serial concentration of NaCl (0.0- 0.8%) is similar to native unloaded cells. From the results of osmotic fragility and hematological parameters, it seems that pravastatin may preserve the physiological and morphological characters of erythrocytes membrane. This suggestion was supported by our previous study stated that this statin preserve of erythrocytes fragility and morphology during drug loading [35].
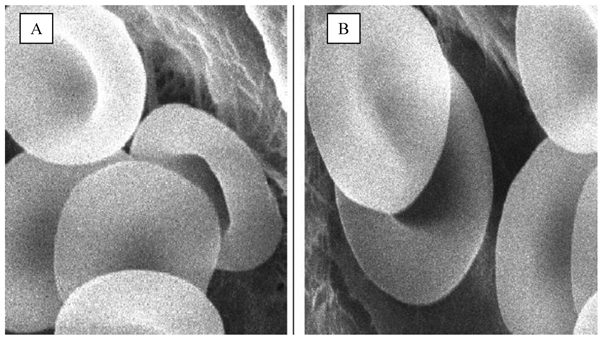
Scanning electron microscopy
In Figure 3, the scanning electron micrographs erythrocytes of drug loaded erythrocytes by our selected method at 5000 magnifications. The loading process resulted in different stages of normal biconcave shape and without changes in morphology. This result shows that the loading process is not aggressive and/or the drug has no deleterious effects on erythrocyte shape. The maintenance of carrier cells morphology similar to native cells gives the opportunity for carrier erythrocytes to life span like native [19]. This result suggested that loaded erythrocytes can be used for sustained release of pravastatin. This assumption is supported by previous findings which recommended that the use of carrier erythrocytes as extended release system for prednisolone due to the minimal effect of drug loading on the erythrocytes morphology[13].
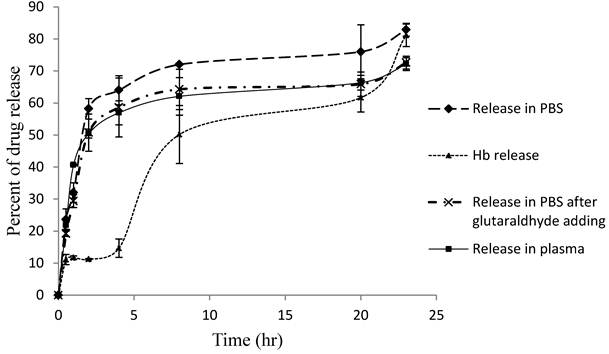
Pravastatin release
Figure 4 shows that the pravastatin release pattern from loaded erythrocytes in PBS, plasma and PBS after using glutaraldehyde as a membrane stabilizer. Also the figure shows the hemoglobin release. The releasing curves of pravastatin show 2 release phases, the first is rapid release phase and the second is slow sustained release phase with hemoglobin release similar pattern. Pravastatin release in PBS is rapid process compared to release in plasma and after glutaraldehyde treatment. It reaches 83% in PBS after 23 hours, and then decreased to 72% after treat with glutaraldehyde after 23 hours. While in plasma it reaches only 72% after 23 hours. These results are comparable to results reported on similar polar drugs including gentamicin[38], enalaprilat [39] and heparin [40]. Hemoglobin release kinetics is belonged to zero order pattern while pravastatin is belonged to first order kinetics in both two phases. The two phases may be, first, due to coupling the drug to erythrocyte membrane [36] as predicted before. Second, it may be due to the presence of some efflux transporters in erythrocyte membrane mediating drug active efflux out of the cell [15].
Scanning electron micrograph of pravastatin loaded erythrocytes by endocytosis. A) Control erythrocytes, B) Pravastatin loaded erythrocytes, morphological features like the control one. Magnification is X5000.
Percent of pravastatin and hemoglobin release from loaded erythrocytes in PBS and plasma. Data were tested by one-way analysis of variance and represented as mean ± SD. Three samples in each group (N = 3). Bonferroni multiple comparison tests using SPSS software was performed to determine differences between mean values at (P ≤ 0.01).
Conclusion
The endocytosis method is less destructive to erythrocytes and preserves the cells fragility and morphology like the native ones. Furthermore, pravastatin has no deleterious effect on erythrocyte as indicated by osmotic fragility test and SEM. The results revealed that pravastatin was loaded successfully on human erythrocytes with acceptable loading parameters. Pravastatin releases from loaded erythrocytes obeying first order kinetics and it needs 24 hours to release 71% of loaded drug in plasma. These results suggested that erythrocyte is suitable carrier for retarded released pravastatin. However, the relative impacts of different in vitro findings on the overall in vivo drug delivery efficacy of these cellular carriers remain to be evaluated during the future in vivo studies.
Acknowledgements
The authors would like to thank Dr. Walid Al-Kayyali chair for Pharmaceutical industries for the assistance in completion of this work, Also, Dr. Magdy Abdelhamid for his kind helping and efforts in drug analysis.
This Work was funded by Deanship for Scientific Research (NPAR3-(2)), and by SABIC graduate student fund (MED-30-41).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. McFarlane SI, Muniyappa R, Francisco R, Sowers JR. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J Clin Endocrinol Metab. 2002;87(4):1451-1458
2. Moghadasian MH. Clinical pharmacology of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Life Sciences. 1999;65(13):1329-1337
3. Williams D, Feely J. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin Pharmacokinet. 2002;41(5):343-370
4. Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397-412
5. Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2004;57(2):181-187
6. Koitabashi Y, Kumai T, Matsumoto N, Watanabe M, Sekine S, Yanagida Y, Kobayashi S. Orange juice increased the bioavailability of pravastatin, 3-hydroxy-3-methylglutaryl CoA reductase inhibitor, in rats and healthy human subjects. Life Sciences. 2006;78(24):2852-2859
7. Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am Heart J. 2006;151(2):273- 281
8. Oda S, Nagahama R, Nakano K, Matoba T, Kubo M, Sunagawa K, Tominaga R, Egashira K. Nanoparticle-mediated endothelial cell-selective delivery of pitavastatin induces functional collateral arteries (therapeutic arteriogenesis) in a rabbit model of chronic hind limb ischemia. JVS. 2010;52(2):412-420
9. Kang B. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int J Pharm. 2004;274(1-2):65-73
10. Charman WN, Chan HK, Finnin BC, Charman SA. Drug Delivery: A Key Factor in Realising the Full Therapeutic Potential of Drugs. Drug Dev Res. 1999;46:316-327
11. Margulis-Goshen K, Magdassi S. Formation of simvastatin nanoparticles from microemulsion. Nanomedicine: NBM. 2009;5(3):274-281
12. Gupta A, Mishra AK, Bansal P, Kumar S, Gupta V, Singh R, Kalyan GS. Cell Based Drug Delivery System Through Resealed Erythrocyte- A Review. Int J Pharm. 2010;2(1):23-30
13. Shavi GV, Doijad RC, Deshpande PB, Manvi FV, Meka SR, Udupa N, Omprakash R, Dhirendra K. Erythrocytes as carrier for prednisolone: in vitro and in vivo evaluation. Pak J Pharm Sci. 2010;23(2):194-200
14. Gothoskar AV. Resealed Erythrocytes:A Review. J Pharm Technol. 2004:140-158
15. Hamidi M, Zarrin A, Foroozesh M, Zarei N, Mohammadisamani S. Preparation and in vitro evaluation of carrier erythrocytes for RES-targeted delivery of interferon-alpha 2b. Int J Pharm. 2007;341(1-2):125-133
16. Gopal VS, Ranjith Kumar AN. Usha NA, Karthik A, Udupa N. Effective drug targeting by Erythrocytes as Carrier Systems. Curr Trends Biotechnol Pharm. 2007;1(1):18-33
17. Davoust J, Gruenberg J, Howell KE. Two threshold values of low pH block endocytosis at different stages. EMBO J. 1987;6(12):3601-3609
18. Patel RP. An Overview of Resealed Erythrocyte Drug Delivery. J Pharm Res. 2009;2(6):1008-1012
19. Hamidi M, Tajerzadeh H. Carrier erythrocytes: an overview. Drug Deliv. 2003;10(1):9-20
20. Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60(2):286-295
21. Rossi L, Serafini S, Pierige F, Antonelli A, Cerasi A, Fraternale A, Chiarantini L, Magnani M. Erythrocyte-based drug delivery. Expert Opin Drug Deliv. 2005;2(2):311-22
22. Millan C, Zarzuelo Castaneda A, Sayalero Marinero ML, Lanao JM. Drug, enzyme and peptide delivery using erythrocytes as carriers. J Controlled Release. 2004;95(1):27-49
23. Gutierrez Millan C, Zarzuelo Castaneda A, Sayalero Marinero ML, Lanao JM. Factors associated with the performance of carrier erythrocytes obtained by hypotonic dialysis. Blood Cells Mol Dis. 2004;33(2):132-140
24. Gutierrez Millan C, Bax BE, Castaneda AZ, Marinero ML, Lanao JM. In vitro studies of amikacin-loaded human carrier erythrocytes. Transl Res. 2008;152(2):59-66
25. Hamidi M, Zarei N, Zarrin AH, Mohammadi-Samani S. Preparation and in vitro characterization of carrier erythrocytes for vaccine delivery. Int J Pharm. 2007;338(1-2):70-78
26. Kraus A, Roth HP, Kirchgessner M. Supplementation with vitamin C, vitamin E or beta-carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc-deficient rats. J Nutr. 1997;127(7):1290-1296
27. Solomon M, Wofford J, Johnson C, Regan D, Creer MH. Factors influencing cord blood viability assessment before cryopreservation. Transfusion. 2010;50(4):820-830
28. Schrier SL, Junga I, Ma L. Studies on the effect of vanadate on endocytosis and shape changes in human red blood cells and ghosts. Blood. 1986;68:1008-1014
29. Schrier SL, Junga I, Ma L. Requirements of drug-induced endocytosis by intact human erythrocytes. Blood Cells. 1978;4:339-359
30. Matovcik LM, Junga IG, Schrier SL. Drug-induced endocytosis of neonatal erythrocytes. Blood. 1985;65:1056-1063
31. Hamidi M, Zarrin AH, Foroozesh M, Zarei N, Mohammadi-Samani S. Preparation and in vitro evaluation of carrier erythrocytes for RES-targeted delivery of interferon-alpha 2b. Int J Pharm. 2007;341(1-2):125-133
32. Bossa F, Latiano A, Rossi L, Magnani M, Palmieri O, Dallapiccola B, Serafini S, Damonte G, Andriulli A, Annese V. Erythrocytes-mediated delivery of low doses of dexamethasone revert steroid-dependency in ulcerative colitis. a double-blind, sham-controlled study. Digest Liver Dis. 2008;40(1):S44-S44
33. Talwar N, Jain NK. Erythrocyte based delivery system of primaquine: in vitro characterization. J Microencapsul. 1992;9(3):357-364
34. Hamidi M, Tajerzadeh H, Dehpour AR, Rouini MR, Ejtemaee-Mehr S. In vitro characterization of human intact erythrocytes loaded by enalaprilat. Drug Deliv. 2001;8(4):223-230
35. Alanazi F. Pravastatin provides antioxidant activity and protection of erythrocytes loaded Primaquine. Int J Med Sci. 2010;7(6):358-365
36. Pierige F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60(2):286-295
37. Jain S, Jain NK. Engineered Erythrocytes As A Drug Delivery System. Indian J Pharm Sci. 1997;59(6):275-281
38. Erchler HG, Rameis H, Bauer K, Korn A, Bacher S, Gasic S. Survival of gentamicin-loaded carrier erythrocytes in healthy human volunteers. Eur J Clin Invest. 1986;16(1):39-42
39. Hamidi M, Tajerzadeh H, Dehpour AR, Ejtemaee-Mehr S. Inhibition of serum angiotensin-converting enzyme in rabbits after intravenous administration of enalaprilat-loaded intact erythrocytes. J Pharm Pharmacol. 2001;53(9):1281-1286
40. Erchler HG, Gasic S, Bauer K, Korn A, Bacher S. In vivo clearance of antibody-sensitized human drug carrier erythrocytes. Clin Pharmacol Ther. 1986;40(3):300-303
Author contact
![]() Corresponding author: Fars K. Alanazi, Kayyali Chair for Pharmaceutical Industry. afarsedu.sa
Corresponding author: Fars K. Alanazi, Kayyali Chair for Pharmaceutical Industry. afarsedu.sa

 Global reach, higher impact
Global reach, higher impact