3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(3):203-209. doi:10.7150/ijms.8.203 This issue Cite
Research Paper
Expression of Human Globular Adiponectin-Glucagon-Like Peptide-1 Analog Fusion Protein and Its Assay of Glucose-Lowering Effect In Vivo
1. Department of Geriatrics, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310000, PR China
2. Department of Geriatrics, Hangzhou Hospital of Traditional Chinese Medicine, Hangzhou 310000, PR China
3. College of Life Sciences, Zhejiang University, Hangzhou 310000, PR China
Received 2010-11-17; Accepted 2011-3-1; Published 2011-3-4
Abstract
In this study, human globular adiponectin-glucagon-like peptide-1 analog (gAd-GLP-1-A) fusion protein was expressed and its glucose-lowering effect was measured in vivo. We constructed a prokaryotic expression vector PET28a-gAd-GLP-1-A and transformed the vector into Escherichia coli BL21 (DE3). A recombinant fusion protein of about 25KD was expressed from BL21 (DE3) cells after isopropylthio-β-D-galactoside induction. This protein was N-terminal His-tagged gAd-GLP-1-A fusion protein. Most of the protein was expressed in inclusion body. The fusion protein in inclusion body was purified by using High-Affinity Nickel Iminodiacetic Acid Resin and refolded in urea gradient refolding buffer. The refolded protein was incubated with enterokinase to remove the N-terminal His-tag. The fusion protein without His-tag is gAd-GLP-1-A fusion protein, which exhibited significant glucose-lowering effect in diabetic mice.
Keywords: Escherichia coli, Expression, Globular adiponectin, Globular adiponectin-glucagon-like peptide-1 analog fusion protein, Glucagon-like peptide-1 analog
Introduction
Adiponectin is an adipocyte-specific secretory protein that circulates in blood at high concentrations [1]. It plays important roles in regulating insulin sensitivity and blood glucose levels. Current data have suggested that adiponectin is implicated in the pathogenesis of type 2 diabetes [1]. Blood adiponectin levels are markedly reduced in patients with type 2 diabetes [1]. Administration of recombinant adiponectin can improve insulin sensitivity and significantly reduce blood glucose in diabetic mice [1]. Furthermore, adiponectin has been reported to exhibit protective effects against atherosclerosis and have roles in regulating lipid metabolism [1]. Based on these beneficial effects, adiponectin has been generally studied as a promising candidate for the treatment of type 2 diabetes [1]. Adiponectin is a protein of 247 amino acids consisting of four domains, an amino-terminal signal sequence (1-18 amino acid), a variable region (19-41 amino acid), a collagenous domain (42-107 amino acid), and a C-terminal globular domain (globular adiponectin, 108-244 amino acid) [2]. In these four domains, globular adiponectin (gAd), which has been confirmed to have greater potency than full-length adiponectin, has the potential to become a novel therapeutic agent for the treatment of type 2 diabetes [2].
Glucagon-like peptide 1 (GLP-1) is an incretin hormone released from islet α-cell and intestinal L-cells in response to the ingestion of food [3]. It plays an important role in glucose homeostasis and has shown promising effects as a new treatment for type 2 diabetic patients [3]. The main function of GLP-1 is to enhance glucose-dependent insulin secretion [3]. Administration of GLP-1 can increase insulin secretion and reduce blood glucose [3]. GLP-1 also promotes islet β-cell proliferation, suppresses glucagon secretion, reduces hepatic glucose production, inhibit appetite, and slow the rate of gastric emptying [3]. GLP-1 (1-37), the intracellular precursor of GLP-1, is cleaved from proglucagon, and the first six amino acids are subsequently removed from the N terminus to form bioactive peptides [4]. The principal biologically active forms of GLP-1 are: GLP-1 (7-37) and the predominant circulating active form GLP-1 (7-36) amide [4]. In vivo, both peptides have equipotent biological effects [4]. However, the potential for using GLP-1 to lower blood glucose is limited by its very short plasma half-life [5, 6]. This is due to its rapid inactivation by dipeptidyl peptidase IV and by renal clearance. Developing long-acting GLP-1 analogs (GLP-1-A) to circumvent the rapid inactivation and renal clearance of GLP-1 is therefore an important step toward applying them therapeutically [5, 6].
Type 2 diabetes is characterized by insulin resistance and insulin secretion deficiency. At present, there is no a single medication which treats type 2 diabetes by improving both insulin resistance and insulin secretion deficiency. This study was designed to express human globular adiponectin-glucagon-like peptide-1 analog (gAd-GLP-1-A) fusion protein from Escherichia coli strain BL21 (DE3) and investigate its glucose-lowering effect in diabetic mouse model. The GLP-1-A, which should have greater plasma stability and longer biological half-life, was generated by a substitution of glycolamine for alanine at the second site of GLP-1 (7-37) [7].
Materials and medhods
Materials
Male KM mice (weight 18-20g) were provided by Experimental Animal centre, Zhejiang Chinese Medical University (Hangzhou, China). Plasmid vector PET28a and Escherichia coli host strain BL21 (DE3) were obtained from Zhejiang University Institute of Life Sciences (Hangzhou, China). Mouse anti-His-tag monoclonal antibody was purchased from Novagen Company (Germany). Streptozocin was obtained from Sigma Company (USA). High-Affinity Nickel Iminodiacetic Acid (Ni-IDA) Resin and enterokinase were the products of GenScript Corporation (USA). BCA Protein Assay Kit was purchased from Beyotime Institute of Biotechnology (Jiangshu, China).
Construction of recombinant vector PET28a-gAd-GLP-1-A
Recombinant vector PET28a-gAd-GLP-1-A was constructed according to previous method established by our laboratory (Patent No: 200510050844.8) [8]. Briefly, GLP-1-A gene was obtained by designing a mutation in the gene of GLP-1 (7-37). This mutation resulted in the substitution of glycolamine for alanine at the second site of GLP-1 (7-37) peptide. A sequence of nucleotide including 45 bases was used to connect the 3' terminus of GLP-1-A gene and 5' terminus of gAd gene. The product of this nucleotide sequence was a glycine-rich short peptide including 15 amino acids: [N-(Serine-glycine)7- Serine-C], which was used as a linker to connect the N-terminus of gAd and the C-terminus of GLP-1-A. Because the protein produced from plasmid vector PET28a was an N-terminal 6×His-tagged protein, we introduced an enterokinase cleavage site at the 5' terminus of the gene of gAd-GLP-1-A fusion protein, which was used to remove the N-terminus His-tag [9]. The gene encoding the gAd-GLP-1-A fusion protein was cloned into the expression vector PET28a at Nhe I and HindIII sites.
Expression of N-terminal His-tagged gAd-GLP-1-A fusion protein and Western blot analysis
Protein expression: The Escherichia coli BL21 (DE3) transformed with PET28a-gAd-GLP-1-A were spread in Luria-Bertani liquid medium (1% tryptone, 1% NaCl, 0.5% yeast extract, w/v, pH 7.0) supplemented with 80mg Kanamycin /l and cultured overnight at 37°C. Typically, 2mL of overnight grown culture was added to 200mL of medium and incubated with shaking at 37°C until optical density at 600 nm reached 0.4-0.6. Isopropylthio-β-D-galactoside (IPTG) was then added to a final concentration of 0.4mM and bacterial were cultured for additional 4h at 37°C in shaking incubator to induce the His-tagged gAd-GLP-1-A fusion protein expression. Bacterial cells were harvested by centrifugation at 5000 rpm for 10 min at 4°C, washed with 0.1M phosphate-buffered saline (PBS, pH 7.4) for three times. The sediments were resuspended with 0.1 M PBS, sonicated on ice for 30min, and then recentrifuged in order to separate the supernatant and inclusion body. Part of the production was applied to a 12% SDS-PAGE.
Western blot analysis: The supernatant and inclusion body were analyzed by 12% gels SDS-PAGE, and then transferred to a nitrocellulose membrane (1h, 100V). Following transfer, the membrane was blocked in Tris Buffered Saline with Tween-20 containing 50g/L skimmed milk for 2h, and then incubated with mouse anti-His-tag monoclonal antibody for 2h at room temperature. The strips were washed three times with Tris Buffered Saline (5min each time) and then incubated with horseradish peroxidase-conjugated second antibody for 2h, washed again with Tris Buffered Saline as described previously, and finally developed with 5-Bromo-4-Chloro-3-Indolyl Phosphate /Nitro blue tetrazolium solution.
Purification and refolding of N-terminal His-tagged gAd-GLP-1-A fusion protein
The inclusion body were washed in washing buffer I (0.5% Triton X-100, 50mM Tris-HCl, 10mM EDTA, pH 8.0) for three times, and then in washing buffer II (2M urea, 50mM Tris-HCl, 10 mM EDTA, pH 8.0) for two times. The sediment was dissolved in Binding Buffer (5mM imidazole, 0.5M sodium chloride, 20mM Tris, 8M urea, pH 7.9) at 4°C for about 2h. The insoluble materials were removed by centrifugation at 12000g at 4°C for 15 min. The N-terminal His-tagged gAd-GLP-1-A fusion protein was dissolved in the supernatant. The fusion protein was purified by High-Affinity Ni-IDA Resin. The column was equilibrated with 4 bed volumes of Lysis-Equilibration-Wash (LEW) buffer (50mM sodium dihydrogen phosphate, 300mM sodium chloride, pH 8.0), and the cleared sample containing N-terminal His-tagged gAd-GLP-1-A fusion protein was applied to the column, followed by washing with 8 bed volumes of LEW buffer to remove the unbound protein. The target protein was eluted with 5-10 bed volumes of elution buffer (50mM sodium dihydrogen phosphate, 300mM sodium chloride, 250mM imidazole, 8M urea, pH 8.0). At last, fractions containing pure target protein were collected and analyzed by SDS-PAGE.
The purified N-terminal His-tagged gAd-GLP-1-A fusion protein containing 8M urea was then refolded in urea gradient (6, 4, 2, 1 and 0 M) refolding buffer (20mM Tris-HCl, 1mM EDTA, 0.2mM oxidized glutathione, 2mM reduced glutathione, 0.6M L-arginine, 10% glycerin) at 4°C. The buffer was changed every 12h. The protein concentration was measured by BCA Protein Assay Kit. PEG20000 was used to concentrate the refolded protein.
Removal of N-terminal His-tag
The refolded protein was incubated with enterokinase (1U enterokinase was added in 0.5mg refolded protein) at 22°C for 16h to produce gAd-GLP-1-A fusion protein. The digested products were analyzed by SDS-PAGE and Western blot analysis.
Assay of glucose-lowering effect of gAd-GLP-1-A fusion protein
Male KM mice were housed at 23-25°C in a 12-hour light/dark cycle with access to standard powdered mice chow and normal water. The scientific project, including animal care was supervised and approved by Animals Ethics Committee of the Second Affiliated Hospital, Zhejiang University. They were allowed one week to adapt to their environment before the experiment. And then, the mice were randomly divided into three groups: normal control group, diabetic control group, and diabetic treated group. Each group included 8 mice. Diabetes was induced in mice by a single intraperitoneal injection of streptozotocin (150 mg/kg body weight, dissolved in sodium citrate buffer) after overnight fasting [10]. Mice in normal control group were treated with sodium citrate buffer. 72h after injection, the mice with fasting blood glucose higher than 200 mg/dl were considered as successfully diabetic model mice. After overnight fasting, the mice in diabetic treated group were treated with 15mg/kg body weight of gAd-GLP-1-A fusion protein by intraperitoneal injection. The mice in diabetic control group and normal control group were treated with the same volume of normal saline. Blood glucose was respectively measured at 30min, 1h, 1.5h, 2h, 2.5h and 3h after injection.
Statistical analysis
Data were expressed as means ± standard deviations. Data were analyzed using one-way analysis of variance and secondary analysis for significance with the Turkey-Kramer post test. All analyses were performed using SPSS version 11.0 (SPSS Inc., USA). P <0.05 was considered statistically significant.
Results
Expression of N-terminal His-tagged gAd-GLP-1-A fusion protein and Western blot analysis
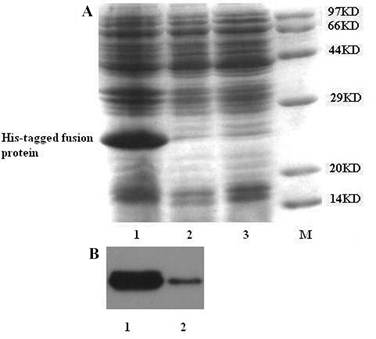
The Escherichia coli host strain BL21 (DE3) cells transformed with the expression vector PET28a-gAd-GLP-1-A produced a recombinant fusion protein of about 25KD after IPTG induction. The protein consists of four domains: 6×His-tag, enterokinase cleavage site (DDDDK), GLP-1-A (31 amino acids), linker (glycine-rich short peptide, 15 amino acids), and gAd (137 amino acids) (Fig. 1A). The fusion protein was absent in non-induced condition. SDS-PAGE analysis showed that most of the fusion protein was in inclusion body (Fig. 2A). Western blot using mouse anti-His-tag monoclonal antibody also proved that majority of fusion protein was present in inclusion body (Fig. 2B).
Maps of N-terminal His-tagged gAd-GLP-1-A fusion protein and gAd-GLP-1-A fusion protein. (A) N-terminal His-tagged gAd-GLP-1-A fusion protein; (B) gAd-GLP-1-A fusion protein.

Expression of N-terminal His-tagged gAd-GLP-1-A fusion protein. Before IPTG induction, part of Escherichia coli BL21 (DE3) transformed with recombinant vector were collected and lysed. The lysate was analyzed by 12% SDS-PAGE. After IPTG induction, the Escherichia coli BL21 (DE3) transformed with recombinant vector were sonicated and centrifuged to separate the supernatant and inclusion body. Part of the production was applied to 12% SDS-PAGE analysis and Western blot analysis. (A) SDS-PAGE analysis: Most of the fusion protein was found in inclusion body. The expected molecular weight of the fusion protein is about 25KD. M: protein molecular weight marker; Lane 1: inclusion body; Lane 2: bacterial cell lysate before IPTG induction; Lane 3: supernatant. (B) Western blot analysis: Mouse anti-His-tag monoclonal antibody was used for this analysis. The fusion protein was observed in both inclusion body and supernatant. But most of them were in inclusion body. Lane 1: inclusion body; Lane 2: supernatant.
Purification and refolding of N-terminal His-tagged gAd-GLP-1-A fusion protein
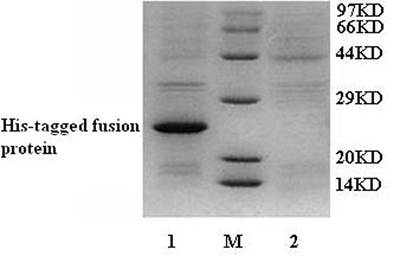
The fusion protein was purified by High-Affinity Ni-IDA Resin. After filtering, the fusion protein was bound in the column. The column was washed by LEW buffer to remove the unbound protein. And then, elution buffer was used to elute the fusion protein. The results were analyzed by 12% SDS-PAGE gel. No fusion protein was found in LEW buffer after washing the column (Fig. 3). However, we detected the fusion protein in elution buffer (Fig. 3). The purified N-terminal His-tagged fusion protein was then refolded by urea gradient refolding buffer.
SDS-PAGE analysis for the purification of N-terminal His-tagged gAd-GLP-1-A fusion protein. M: protein molecular weight marker; Lane 1: purified protein in elution buffer; Lane 2: LEW buffer after washing column.
Enterokinase cleavage of the N-terminal His-tagged gAd-GLP-1-A fusion protein
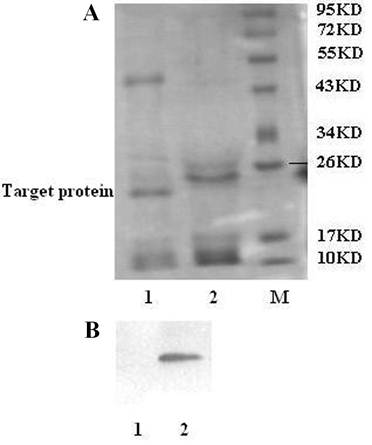
To obtain functional gAd-GLP-1-A fusion protein, the His-tag must be removed from the N-terminal His-tagged gAd-GLP-1-A fusion protein. Enterokinase can recognize the sequence Asp-Asp-Asp-Asp-Lys (DDDDK) and cleave the peptide bond after the lysine residue [9]. The enzyme can cleave any fusion protein that carries this sequence [9]. The N-terminal His-tagged gAd-GLP-1-A fusion protein was incubated with enterokinase to remove the N-terminal His-tag. An approximately 22KD cleavage fragment was observed after the incubation, which was analyzed by SDS-PAGE (Fig. 4A). Western blot did not detect His-tag reactivity after enterokinase cleavage, which suggested that the His-tag was removed from the N-terminal His-tagged gAd-GLP-1-A fusion protein (Fig. 4B). The fusion protein without His-tag was gAd-GLP-1-A fusion protein (Fig. 1B).
Enterokinase cleavage of the N-terminal His-tagged gAd-GLP-1-A fusion protein. (A) SDS-PAGE analysis: After enterokinase cleavage, we observed a cleavage fragment of 22KD. The fragment was gAd-GLP-1-A fusion protein. M: protein molecular weight marker; Lane 1: after enterokinase cleavage; Lane 2: before enterokinase cleavage. (B) Western blot analysis: No His-tag reactivity was detected after cleavage. Lane 1: after enterokinase cleavage; Lane 2: before enterokinase cleavage.
Glucose-lowering effect of gAd-GLP-1-A fusion protein
We investigated the glucose-lowering effect of gAd-GLP-1-A fusion protein in diabetic mice. Blood glucose was respectively measured at 30min, 1h, 1.5h, 2h, 2.5h and 3h after injection the fusion protein. The results showed blood glucose from diabetic treated group was lower than that from diabetic control group. The difference was significant at 2h, 2.5h, and 3h after injection (P<0.05) (Table 1).
Glucose-lowering effect of gAd-GLP-1-A fusion protein
| Groups (n=8) | Blood glucose (mg/dl) | ||||||
|---|---|---|---|---|---|---|---|
| 0h | 0.5h | 1h | 1.5h | 2h | 2.5h | 3h | |
| Normal control group | 131.75±15.50 | 127.63±12.68 | 104.75±20.55 | 98.38±24.44 | 93.88±30.70 | 76.75±33.01 | 75.38±34.99 |
| Diabetic control group | 300.63±104.69a | 244.13±107.03b | 222.75±104.81 b | 201.38±91.64 b | 209.50±87.61 a | 203.75±100.30 a | 180.25±111.82 b |
| Diabetic treated group | 294.13±89.97a | 208.13±76.43 | 170.00±76.91 | 150.13±56.23 | 130.63±47.67c | 92.63±46.12d | 87.88±46.76 c |
a Compared with normal control group P<0.01; b Compared with normal control group P<0.05; c Compared with diabetic control group P<0.05; d Compared with diabetic control group P<0.01
Data were given as means ± standard deviations
Discussion
In the present study, we developed, for the first time, a successful protocol for expression human gAd-GLP-1-A fusion protein from Escherichia coli strain BL21 (DE3). Plasmid vector PET28a was used to express this fusion protein. This vector can produce an N-terminal His-tagged protein. His-tag is often used for protein purification [11]. The affinity of the His-tag for metal ions allows the fusion product to be quickly separated from the bulk of other bacterial proteins by using metal chelate affinity chromatography [11]. Because N-terminal His-tag may influence the function of protein, we designed an enterokinase cleavage site at the 5' terminus of the gene of the gAd-GLP-1-A fusion protein, which was used to remove the His-tag [9]. In our study, most of the His-tagged fusion protein expressed from BL21 (DE3) was present in inclusion body. In order to recover its function, the fusion protein in inclusion body was refolded in urea gradient refolding buffer. And then, the refolded protein was incubated with enterokinase to remove the His-tag. The fusion protein without His-tag is gAd-GLP-1-A fusion protein, which exhibited significant glucose-lowering effect in diabetic mice.
GLP-1 has been reported as a promising therapeutic agent for type 2 diabetes [3, 12]. However, the clinical application of native GLP-1 is hampered by its very short plasma half-life [5, 6, 13]. This is due to its rapid inactivation by dipeptidyl peptidase IV and by renal clearance [5, 6, 13]. Many attempts have been made to increase its biological half-life and its efficacy in vivo by producing dipeptidyl peptidase IV-resistant GLP-1 analogs via amino acid substitution and hindering the renal clearance of GLP-1 by conjugating it to other molecules [5, 6, 13]. Circulating GLP-1 is inactivated after cleaving the first two amino acids at the N-terminus by dipeptidyl peptidase IV [14]. Studies reported that the replacement of alanine with glycine at the second site of GLP-1 could increase the resistance of GLP-1 on dipeptidyl peptidase IV mediated degradation [7]. This change is sufficiently subtle to retain the biological activity of GLP-1 [7]. Moreover, GLP-1 is a peptide with relatively low molecular weight and small molecular size, and most of them may not connect with plasma albumin [15]. These characteristics facilitate the filtration of GLP-1 through kidney [15]. Although structural modification of GLP-1 may overcome degradation by dipeptidyl peptidase IV, this does not address the loss of GLP-1 by renal filtration [5]. Conjugating GLP-1 to other molecular may prevent renal filtration of GLP-1 [5, 6]. Adiponectin is an adipocyte-specific secretory protein and plays important roles in regulating insulin sensitivity and blood glucose levels [1]. The plasma half-life of adiponectin is very long, about 2.5-6h [16]. Adiponectin consists of four domains [2]. The gAd is its functional domain [2]. No study has reported the half-life of gAd. However, gAd has been confirmed to have greater biological activity than full-length adiponectin. We selected gAd as the conjugating molecule of GLP-1 in our study. This design not only may prevent the renal filtration of GLP-1, but also may yield a new protein with both function of GLP-1 and gAd [2].
We designed a mutation in the gene of GLP-1 (7-37) in the present study. This mutation resulted in the substitution of glycolamine for alanine at the second site of GLP-1 (7-37) peptide. Study has reported that glycine-rich linker is flexible, which allows the specific engineering of hinge regions into proteins to achieve desired functional motions [17]. We used a glycine-rich short peptide including 15 amino acids to connect the N-terminus of globular adiponectin and the C-terminus of GLP-1-A. Compared with native GLP-1, the fusion protein has a modified site and larger molecular size, and may circumvent the rapid inactivation and renal clearance of GLP-1. Studies have reported N-terminus is very important for the biological activity of GLP-1, and for globular adiponectin, the C-terminus is important [2, 5, 14]. Thus, we connected the N-terminus of globular adiponectin and the C- terminus of GLP-1-A through the linker, which could make the N-terminus of GLP-1-A and the C-terminus of gAdiponectin be free and interact productively with their receptor on target cells.
In summary, we have succeeded in expressing the human gAd-GLP-1-A fusion protein from Escherichia coli BL21 (DE3). This fusion protein exhibited significant glucose-lowering effect in diabetic mice and may be a promising agent that can treat type 2 daibetes by improving both insulin resistance and insulin secretion deficiency. However, we only observed the glucose-lowering effect of the whole fusion protein in this study. We could not determine which part of the fusion protein has this effect. The effect might due to either one part of fusion protein or both of them. In other words, we need to know whether each part of the fusion protein play glucose-lowering effect separately. We also need to know whether the half-life of the GLP-1-A is longer than native GLP-1 as well as whether fusion protein can exhibit other functions of both gAd and GLP-1. Additional experiments should be performed to fully investigate the function and characteristics of the fusion protein in the future.
Acknowledgements
This work was supported by research grant from the National Natural Science Foundation of China (No: 30671007, 30300165) and the grant from the Traditional Chinese Medicine Administration of Zhejiang Province, China (No: 2010ZB075).
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Menzaghi C, Trischitta V, Doria A. Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;5:1198-209
2. Hu XB, Zhang YJ, Zhang HT. et al. Cloning and expression of adiponectin and its globular domain, and measurement of the biological activity in vivo. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2003;11:1023-8
3. Gautier JF, Choukem SP, Girard J. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab. 2008;34(Suppl 2):S65-S72
4. Vahl TP, Paty BW, Fuller BD. et al. Effects of GLP-1-(7-36)NH2, GLP-1-(7-37), and GLP-1- (9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab. 2003;4:1772-9
5. Green BD, Lavery KS, Irwin N. et al. Novel glucagon-like peptide-1 (GLP-1) analog (Val8)GLP-1 results in significant improvements of glucose tolerance and pancreatic beta-cell function after 3-week daily administration in obese diabetic (ob/ob) mice. J Pharmacol Exp Ther. 2006;2:914-21
6. Chen J, Bai G, Cao Y. et al. One-step purification of a fusion protein of glucagon-like peptide-1 and human serum albumin expressed in pichia pastoris by an immunomagnetic separation technique. Biosci Biotechnol Biochem. 2007;11:2655-62
7. Deacon CF, Knudsen LB, Madsen K. et al. Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia. 1998;3:271-8
8. Tongfeng Zhao, Zhan Yuhong, Gu Wei. Construction of human globular adiponectin-glucagons-like peptide-1 fusion protein expression vector. Chongqing Medical. 2006;14:1251-54
9. LaVallie ER, Rehemtulla A, Racie LA. et al. Cloning and functional expression of a cDNA encoding the catalytic subunit of bovine enterokinase. J Biol Chem. 1993;31:23311-7
10. Cai L, Li W, Wang G. et al. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;6:1938-48
11. Hengen P. Purification of His-Tag fusion proteins from Escherichia coli. Trends Biochem Sci. 1995;7:285-6
12. Yu BS, Wang AR. Glucagon-like peptide 1 based therapy for type 2 diabetes. World J Pediatr. 2008;1:8-13
13. Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;22:2471-83
14. Elahi D, Egan JM, Shannon RP. et al. GLP-1 (9-36) amide, cleavage product of GLP-1 (7-36) amide, is a glucoregulatory peptide. Obesity (Silver Spring). 2008;7:1501-9
15. Ruiz-Grande C, Alarcón C, Alcántara A. et al. Renal catabolism of truncated glucagon-like peptide 1. Horm Metab Res. 1993;12:612-6
16. Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to i nsulin sensitivity. Obes Rev. 2005;1:13-21
17. Wriggers W, Chakravarty S, Jennings PA. Control of protein functional dynamics by peptide linkers. Biopolymers. 2005;6:736-46
Author contact
![]() Corresponding author: Tongfeng Zhao, Ph.D., Department of Geriatrics, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310000, PR China. Tel: 86-571-887783690; Fax: 86-571-87022660; e-mail: zhaotongfengcom.cn
Corresponding author: Tongfeng Zhao, Ph.D., Department of Geriatrics, the Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310000, PR China. Tel: 86-571-887783690; Fax: 86-571-87022660; e-mail: zhaotongfengcom.cn

 Global reach, higher impact
Global reach, higher impact