3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2011; 8(1):74-83. doi:10.7150/ijms.8.74 This issue Cite
Research Paper
Intravenous transplantation of allogeneic bone marrow mesenchymal stem cells and its directional migration to the necrotic femoral head
1. Department of Orthopaedics, Renmin Hospital of Wuhan University, Wuhan 430060, China.
2. Department of Orthopedics, Affiliated Hospital of Hebei University, Baoding 071000, China.
3. Laboratory of Biochemistry and Molecular Biology, Institute of Basic Medical Sciences, Academy of Military Medical Sciences, Beijing 100850, China.
4. College of Health Science, Wuhan Institute of Physical Education, Wuhan 430079, China.
* Zhang-hua Li and Wen Liao contributed equally to this work.
Received 2010-8-11; Accepted 2011-1-1; Published 2011-1-9
Abstract
In this study, we investigated the feasibility and safety of intravenous transplantation of allogeneic bone marrow mesenchymal stem cells (MSCs) for femoral head repair, and observed the migration and distribution of MSCs in hosts. MSCs were labeled with green fluorescent protein (GFP) in vitro and injected into nude mice via vena caudalis, and the distribution of MSCs was dynamically monitored at 0, 6, 24, 48, 72 and 96 h after transplantation. Two weeks after the establishment of a rabbit model of femoral head necrosis, GFP labeled MSCs were injected into these rabbits via ear vein, immunological rejection and graft versus host disease were observed and necrotic and normal femoral heads, bone marrows, lungs, and livers were harvested at 2, 4 and 6 w after transplantation. The sections of these tissues were observed under fluorescent microscope. More than 70 % MSCs were successfully labeled with GFP at 72 h after labeling. MSCs were uniformly distributed in multiple organs and tissues including brain, lungs, heart, kidneys, intestine and bilateral hip joints of nude mice. In rabbits, at 6 w after intravenous transplantation, GFP labeled MSCs were noted in the lungs, liver, bone marrow and normal and necrotic femoral heads of rabbits, and the number of MSCs in bone marrow was higher than that in the, femoral head, liver and lungs. Furthermore, the number of MSCs peaked at 6 w after transplantation. Moreover, no immunological rejection and graft versus host disease were found after transplantation in rabbits. Our results revealed intravenously implanted MSCs could migrate into the femoral head of hosts, and especially migrate directionally and survive in the necrotic femoral heads. Thus, it is feasible and safe to treat femoral head necrosis by intravenous transplantation of allogeneic MSCs.
Keywords: femoral head necrosis, bone marrow mesenchymal stem cell, migration, safety
Introduction
Recently, stem cell transplantation has been a focus in the treatment of some diseases. Stem cells have the potential of multi-directional differentiations, and they can differentiate into specialized cells to repair injured tissues under certain conditions [1]. Animal experiments have demonstrated that in anoxic environment, implanted stem cells can differentiate and promote neovascularization which effectively increase the blood perfusion in ischemic tissues, and thus inhibit further necrosis of tissues [2,3]. Researchers have transplanted the bone marrow stem cells into the necrotic femoral heads, and results show bone marrow stem cells can remove vascular lesions and promote angiogenesis in necrotic femoral heads, accompanied by significant improvement of blood circulation in the necrotic femoral head and surrounding tissues [4]. Mesenchymal stem cells (MSCs) are multipotent stem cells that can differentiate into a variety of cell types. In the field of cell transplantation, MSCs have many advantages over other cell types such as easy isolation and culture, rapid in vitro amplification, differentiation potential, and easy collection [2]. Currently, MSCs have been applied in the treatment of femoral head necrosis. Experiments demonstrate the implanted MSCs can not only survive but proliferate in the necrotic femoral head after transplantation, promoting the repair of injured femoral head [5]. In addition, intravenously implanted MSCs can migrate into and repair the injured tissues [6,7]. Thus, allogeneic transplantation of MSCs through intravenous injection may be a minimally invasive strategy for the treatment of femoral head necrosis. In this study, green fluorescent protein (GFP) labeled allogeneic MSCs were intravenously injected into nude mice and the distribution and migration of MSCs were dynamically monitored to evaluate the feasibility and safety of intravenous implantation of allogeneic MSCs in the treatment of femoral head necrosis. Our study may provide theoretical basis for the clinical application of MSCs.
Materials and methods
Reagents and instruments
In the present study, L-DMEM medium, fetal bovine serum (Hyclone, USA), Percoll separating medium (Sigma, USA), Kodak DXS small animal imaging system and adenovirus vector carrying GFP (Adeasy GFP) were used. The adenovirus vector carrying GFP was kindly provided by Professor Zhou (Peking University, China).
Experimental animals
A total of 12 male rabbits (6 months old) weighing 2.5 ± 0.5 kg and 18 nude mice (6 weeks old) weighing 15-20 g were purchased from the Experimental Animal Center, Academy of Military Medical Sciences, China. This study was approved by the Ethics Committee of our university.
Amplification and purification of Adeno-GFP recombinant adenovirus vector
Adeno-GFP infected HEK293 cells and their lysate were harvested when cytopathogenic effects appeared. After three freeze-thaw cycles, solution was centrifuged at 14000 g for 10 min, and viruses were harvested from the supernatant. Viruses were purified by cesium chloride density gradient centrifugation, and virus titer was determined after the formation of virus negative colonies. Virus vectors were preserved at -80 ºC.
Isolation, purification, culture and identification of rabbit MSCs
Heparin anti-coagulated bone marrow was collected from the rabbit right proximal tibia under sterile conditions. MSCs were isolated by density gradient centrifugation with Percoll. Then, these cells were re-suspended in L-DMEM culture medium containing 10% fetal bovine serum, 100 U/ml streptomycin and 100 U/ml penicillinum at a density of 2.0×105/cm2 and incubated in 25 cm2 flasks at 37 ºC in humidified atmosphere with 5% CO2. After 3 days of culture, the medium was refreshed, and then the medium changed every other day. Cell passaging was performed when cell confluence reached 85%. The purity and immunophenotype of MSCs and their potentials of osteogenesis and adipogenesis were determined.
Femoral head necrosis animal model
The rabbit model of femoral head necrosis was established according to previously reported [8]. Weight loading area of femoral heads was exposed and treated with liquid nitrogen for 3-5 min until the articular cartilage of femoral head became pale. Immediately, femoral head was re-warmed with normal saline at 37 ºC for 3 min. Then, the wound was closed and covered with sterile dressing, and 800 000 U of penicillin were intramuscularly administered for each rabbit immediately followed by 400 000 U of penicillin daily for consecutive 5 days.
Cell labeling and transplantation
In order to observe the distribution of implanted MSCs in vivo, MSCs were labeled with GFP in vitro before transplantation [9]. In brief, the solution containing GFP was added to MSCs followed by incubation for 6 h. Then, low glucose DMEM containing 10% serum of equal volume was added followed by incubation for 72 h. The transfection efficiency was detected under a fluorescence microscope. A total of 5×105 GFP-labeled MSCs (about in 300 μl of cell suspension) were injected into nude mice through vena caudalis. At 0, 6, 24, 48, 72 and 96 h after transplantation, the nude mice were anesthetized and placed in a supine position. The in vivo GFP-labeled MSCs were dynamically monitored in a Kodak DXS small animal imaging system [10]. When, the anesthetized nude mice were placed on the platform, the background image was taken under the lights of an illuminator. Then, the illuminator was turned off, and the image of light emitted from the nude mice, namely bioluminescence image, was taken. Then, two images were merged and the location of light source was shown in mice.
At 2 w after femoral head necrosis, 5×107 GFP-labeled MSCs (about in 3 ml of cell solution) were injected into rabbits through the ear vein, within more than 1 min. Necrotic and normal femoral heads, bone marrows, lungs and livers were harvested at 2, 4 and 6 w after transplantation and sectioned followed by observation under a fluorescent microscope. At 24 h, 72 h, 1 w, 4 w and 6 w after transplantation, the manifestations of immunological rejection and graft versus host disease were monitored.
Statistical analysis
Three sections were used for analysis. Five fields from each section were randomly selected and GFP positive cells were counted at a magnification of 200. Data were expressed as means ± standard deviation (SD). Statistical analysis was performed with SAS6.12 statistic software package and Student t test was carried out for comparisons. A value of P<0.05 was considered statistically significant.
Results
Observation of immunological rejection and graft versus host disease
During the experiment, all animals survived. There were no significant changes in the heart rate, breath rate, body temperature, mental condition, urination, and defecation. Routine blood tests and tests of liver or renal functions showed normal. No acute or chronic toxicity and manifestations of graft versus host disease were observed. Besides, no swelling at injection sites and lower limb movement disorder were noted.
Isolation, culture, identification and GFP labeling of MSCs
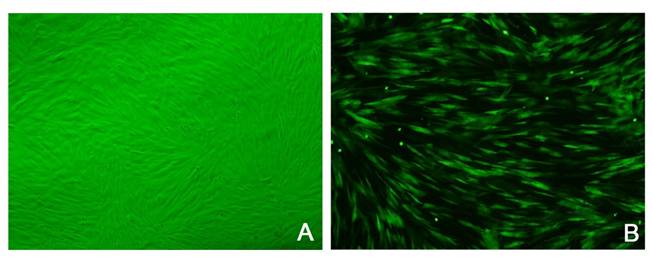
At early stage, rabbit MSCs were long spindle-shaped and fibroblast-like, and arranged parallelly. Subsequently, a majority of MSCs gradually presented whirl-like growth (Figure 1A), and a small amount of rabbit MSCs were polygonal. After Adeno-GFP infection, the morphology, and proliferation of cells were not significantly changed (Figure 2). At 24 h after infection, scattered green fluorescence was observed under fluorescence microscope and bright green fluorescent observed at 72 h after infection (Figure 1B). The infection rate was over 70%.
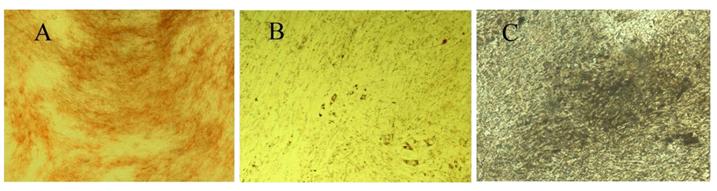
Flow cytometry showed the MSCs had no expressions of CD34, CD45 and HLA-DR, but high expressions of CD29 and CD44. Furthermore, the purity of cells with these phenotypes was as high as 99% which suggested the homogeneous phenotype. After adipogenesis induction for 1 week, oil red O staining showed lipid droplets in the MSCs. After osteogenesis induction for 1 week, alkaline phosphatase staining showed positive cells, and 3 weeks after osteogenesis induction, bone nodule was present demonstrated by VonKossa staining (Figure 3 A, B, C).
MSCs after isolated culture. a: MSCs of passage 3 under a light microscope (×100); b: MSCs labeled with GFP under fluorescent microscope(×100).
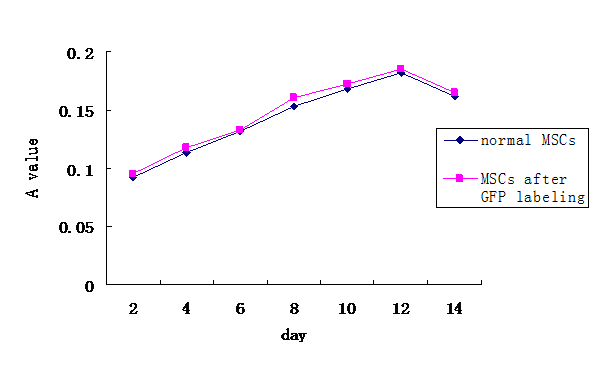
Cell proliferation curve. The proliferation of Adeno-GFP infected MSCs was similar to that of normal MSCs.
Induction of osteogenesis and adipogenesis of MSCs (×100). A: One week after osteogenesis induction, alkaline phosphatase staining showed positive cells. B: One week after adipogenesis induction, oil red O staining showed lipid droplets in the MSCs. C: Three weeks after osteogenesis induction, bone nodule was present demonstrated by VonKossa staining.
Distribution of MSCs in vivo
Kodak DXS small animal imaging system is a real-time imaging system which can be used to observe the distribution of cells in living animals in a real time pattern. In the imaging system, GFP fluorescence presented bright white. Most implanted MSCs concentrated in the tail of nude mice immediately after transplantation, and a small amount of MSCs were distributed in the right hip joint. Subsequently, MSCs migrated into almost all organs, and were uniformly distributed in the brain, lungs, heart, kidney, intestine, hip joints and other organs at 24 h after transplantation. At 48 h after transplantation, the amount of MSCs in tissues gradually decreased, and nearly no GFP fluorescence was observed in nude mice at 96 h after transplantation. These findings indicate that intravenously injected MSC could migrate into the femoral head and stayed in the femoral head for a relatively long time (Figure 4 A-F).
Gross presentations of femoral head
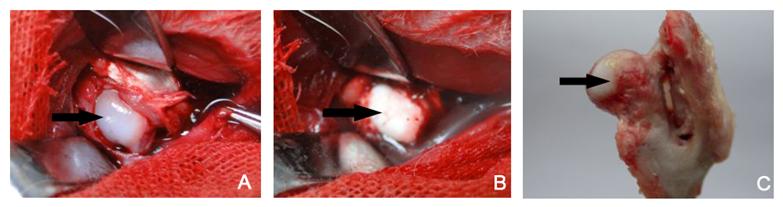
The surface of normal femoral head was smooth and round, and the articular cartilage was transparent and glossy (Figure 5A). After surgery, the shape of femoral head was not markedly changed and the surface of femoral head was pale and dull without normal glossiness and smoothness. The transparency was decreased (Figure 5B). At 6 w after MSC transplantation, the shape of femoral head was integrity and the articular cartilage largely preserved. The articular cartilage was glossy and smooth, a fraction of which present dark red (Figure 5C).
Distribution of GFP positive cells in nude mice
After blue excitation light was absorbed, GFP presented green fluorescence. At 6 h after intravenous allogeneic MSC implantation, GFP-labeled MSCs were observed in the lungs, liver, bone marrow and normal femoral head, and the amount of GFP positive MSCs in the bone marrow was higher than that in the liver, lungs and femoral head. There were also a lot of GFP labeled MSCs in the necrotic region of femoral head at different time points, and the number of cells presenting green fluorescence reached a maximal level at 6 w after transplantation, indicating that intravenously implanted GFP-labeled MSCs can migrate into multiple tissues with circulation of blood flow. MSCs could directionally migrate into and survive in the necrotic area of femoral heads (Table 1 and Figure 6).
In vivo migration of MSCs after transplantation. A: immediately after intravenous MSCs transplantation; B: 6 h after MSCs transplantation; C: 24 h after MSCs transplantation; D: 48 h after MSCs transplantation; E: 72 h after MSCs transplantation; F: 96 h after MSCs transplantation.

Gross presentations of normal and necrotic femoral heads. A: Femoral head before necrosis; B: Femoral head immediately after necrosis; C: Femoral head at 6 w after MSCs transplantation. Black arrow shows the surface of femoral head. The normal femoral head was smooth and round, and the articular cartilage was transparent and glossy (A). After freezing, the femoral head was pale in the absence of normal glossiness and smoothness (B). A fraction of articular cartilage was dark red (C).
GFP positive MSCs in different tissues after intravenous transplantation under fluorescence microscope. a: Lung; b: Liver; c: bone marrow; d: normal femoral head; e: necrotic femoral head at 2 w after MSCs transplantation; f: necrotic femoral head at 4 w after MSCs transplantation; g: necrotic femoral head at 6 w after MSCs transplantation. Green cells were GFP positive MSCs. Figures a'-g' were sections under light microscope.
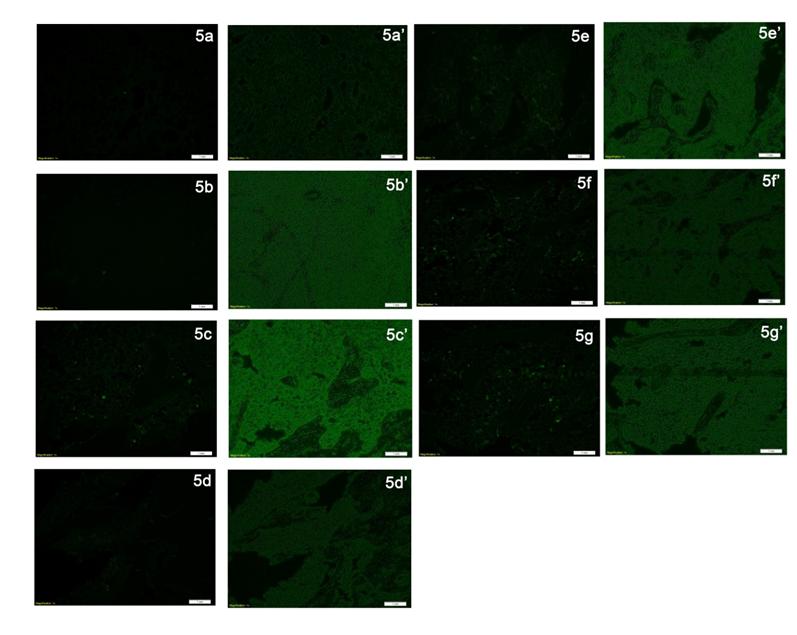
Number of GFP-labeled MSCs in different tissues of rabbits at different time points (n=3)
| Tissues | 2w | 4w | 6w |
|---|---|---|---|
| Lungs | 22.67±1.53 | 18.67±1.53 | 11.67±1.53 |
| Liver | 26.67±1.53 | 19.67±1.53 | 13.33±1.53 |
| Bonemarrow | 40.00±4.36 | 29.00±1.00 | 23.33±1.53 |
| Normal femoral head | 12.67±1.53 | 9.67±0.58 | 6.33±0.58 |
| Necrotic femoral head | 26.33±0.58 | 49.33±2.52*# | 66.33±3.51*# |
Note: * P<0.05 vs 2 w; # P<0.05 vs 2 w and 4 w.
Discussion
At present, intravenous transplantation has been a common strategy in the stem cell transplantation and researchers have applied it in the treatment of a lot of diseases including severe autoimmune diseases (systemic lupus erythematosus, multiple sclerosis, rheumatoid arthritis, etc) [11-14], myocardial infarction [15-17], liver failure [18], trauma [19-21]. Recently, in order to improve the efficacy of stem cell transplantation, committed stem cells are isolated and purified, and single lineage stem cell transplantation is then performed. Deng et al applied intravenous infusion of MSCs in the treatment of spinal cord injury [6]. Lange et al treated the acute renal failure by intravenous infusion of MSCs [7].
Ischemic necrosis is the most common type of femoral head necrosis. Ischemic femoral head necrosis refers to a disease which results from interruption of blood supply to the femoral head resulting in ischemia, necrosis and collapse of femoral head. This disease is frequently found in middle-aged adults and leads to serous hip joint dysfunction. Ischemic femoral head necrosis has been one of common but refractory diseases. The clinical treatments of ischemic femoral head necrosis include: (1) Non-surgical treatments [22-24]: pharmacotherapy, extracorporeal shock wave therapy, hyperbaric oxygen, interventional therapy, etc. The efficacy of non-surgical treatments is uncertain and different strategies have distinct efficacy. With the development of imaging technique, molecular biology technique and physical therapy, great progress has been made in the non-surgical treatments ischemic femoral head necrosis. (2) Palliative surgery [25-29]: bone grafting, vascular grafting, sequestrum removal+bone tamponade, core decompression surgery, etc. The efficacy of these strategies is inconsistent and they have disadvantages of difficult manipulation. In addition, surgery may cause new trauma and increase the therapeutic cost. (3) arthroplasty [30,31]: Currently, the efficacy of arthroplasty has been significantly improved. However, nowadays, a lot of younger adults develop ischemic femoral head necrosis, and lifetime of prosthesis, risk of surgery and high cost for surgery limit its application in a majority of patients. The abovementioned strategies have limitations and thus it is imperative to develop non-invasive or minimally invasive strategies with high therapeutic efficacy. Recently, the progress in the therapeutic application of stem cells provides promise for the treatment of ischemic femoral head necrosis. When compared with vascular intervention and local drilling for injection, transplantation with stem cells has advantages of minimally invasive and simple manipulation. Therefore, in recent years, a lot of physicians apply stem cell transplantation in the treatment of femoral necrosis [32-37]. However, intravenous injection of stem cells as a therapeutic strategy is less investigated in the treatment of femoral head necrosis.
Safety is a critical concern of intravenous transplantation of MSCs. Whether intravenous transplantation of MSCs can cause immunological rejection? Studies on the immunogenicity of bone marrow MSCs reveal that MSCs can not only avoid the immunological rejection in autologous transplantation, but also reduce the immunological rejection in allogeneic transplantation by inhibiting cell proliferation. Lazarus et al intravenously injected bone marrow MSCs of different concentrations into volunteers, and results showed transplantation of even up to 5×107 MSCs did not cause obvious immunological rejection [38]. Moreover, MSCs can also regulate the secretion of TNF-α, IFN-α, IL-4, and IL-10 and modulate Treg cells to reduce the incidence of graft-versus-host disease. In addition, inhibition or restriction of these inflammatory mediators also alleviates further damage to the bone, cartilage and blood supply to necrotic femoral head [39]. Liu et al conducted a phase I clinical trial to evaluate the feasibility and safety of intravenous transplantation of stem cells. In their study, MSCs were isolated from rhesus monkey and humans in vitro[40]. The purified MSCs of passage 3 were injected into rhesus monkeys and volunteers, independently. During the injection, the vital signs were normal. Before and after injection, the subjects received routine blood examination, routine bone marrow examination, examinations of liver and renal functions and lymphocyte subset. Their results revealed that intravenous transplantation of MSCs was safe and feasible. Devine et al intravenously injected autologous and allogeneic MSC into baboons, and no toxic reactions were observed during 1-year follow-up [41]. Another intravenously injected allogeneic macaque MSCs into macaques or MSCs were directly injected into bone marrow [42]. No abnormalities in routine blood examination and liver and renal functions, no manifestations of acute and chronic toxic reactions and graft versus host disease, no local swelling at injection sites, and no lower limb movement disorder were found during the 2-month follow-up. Nevertheless, the safety of intravenously implanted allogeneic MSCs with high purity should be further confirmed. In the present study, no local or systematic manifestations of acute and chronic toxic reactions and graft versus host disease were observed during and after MSC transplantation. Meanwhile, the body temperature, routine blood parameters and liver and renal functions were shown normal. These findings demonstrate that intravenous transplantation of allogeneic MSCs with high purity is feasible and safe.
Another concern of intravenous transplantation of MSCs is whether the MSCs can migrate into and proliferate in the target tissues, which is the basis of therapeutic effects of MSCs. In adults, MSCs remain the potentials of multi-directional and multi-functional differentiation, and MSCs mainly exist in "storage pool" such as bone marrow, periosteum, blood vessels and loose connective tissue, and play important roles in the repair following tissue injury. MSCs may be motivated to participate in the repair of injured tissues through blood circulation especially after ischemia, trauma, and irradiation [43-46]. At present, it is recognized that the stem cell homing is executed in two ways: (1) Cell necrosis after trauma induces the release of a series of signal molecules, and stem cells are motivated and migrate into peripheral blood and target tissue, in which specific receptors or ligands expressed in injured tissues play important roles. (2) Stem cells circulate among tissues, and stem cells migrate to the injured tissues once injury occurs. Stem cell homing is a complicated process in which a lot of molecules were involved. Once tissues were ischemic, stem cells in circulation are adherent to the vascular endothelial cells, cross the endothelial cells, migrate and finally reached at ischemic sites. Inflammatory may be observed in the local ischemic tissues, and thus a lot of chemotatic factors including interleukin-8 (IL-8), monocyte chemoattractant protein (MCP-1), stromal cell-derived factor (SDF-1) and tumor necrosis factor (TNF) are produced. Meanwhile, the expressions of a variety of adhesion molecules are also up-regulated in vascular endothelial cells [47]. These changes in the micro-environment may contribute to the stem cell homing, which is named by Helmuth et al [48] as “the call of injured tissues for stem cells”. Similarly, in order to confirm that intravenously implanted allogeneic MSCs can migrate to the femoral head, two experiments were carried in this study. First, the distribution of allogeneic MSCs in living nude mice was dynamically monitored after MSC injection. Results showed MSCs can not only migrate into the femoral head, but also retain in the femoral head for a relatively long time. Second, the sections of bone marrow, lungs, liver, and normal and necrotic femoral heads of rabbits with MSC transplantation were observed under fluorescence and light microscope. Results revealed the amount of MSCs in the necrotic femoral head was higher than that in the normal femoral head, liver and lungs, indicating that femoral head ischemia or necrosis can call MSCs to migrate into and survive in injured femoral heads. The above-mentioned findings provided evidence on the curative effects of allogeneic MSC transplantation on ischemic femoral head necrosis.
In the intravenous transplantation of stem cells, it is very important to observe the survival and curative effects of transplanted stem cells. Traditional immunohistochemical method can easily identify the transplanted cells with specific morphology and tissue-specific antigens. However, transplanted MSCs in targeted tissues present normal cell morphology and may be absent of specific markers. Thus it is difficult to determine the implanted allogeneic cells at injured sites. Therefore, cells should be labeled in vitro. An ideal labeling method in vitro must possess high sensitivity and specificity, and long half-life. At present, there are a lot of labeling methods including GFP labeling, Lacz labeling, BrdU labeling, Y chromosome labeling and DiI labeling. GFP protein is stable. GFP gene can be transfected into MSCs through adenovirus vector, resulting in stable GFP expression in MSCs. Although the half-life of GFP is relatively short (4-6 weeks), it is enough to trace the migration of implanted cells during the process of bone formation.
Although our results showed intravenously implanted allogeneic MSCs could directionally migrate to femoral heads, and survive especially in the necrotic femoral heads, the mechanisms underlying the directional migration of MSCs should be further studied. Besides, the efficacy of intravenous transplantation of MSCs in the treatment of ischemic femoral head necrosis should also be further confirmed.
Acknowledgements
The study was supported by the National Natural Science Foundation of China (No. 30700854, 81071463). We greatly appreciate Mr. Qianglin Duan from Tongji Hospital of Tongji University for critical reading of the manuscript.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Noel D, Djouad F, Jorgense C. Regenerative medicine through mesenchymal stem cells for bone and cartilage repair. Curr Opin Investig Drugs. 2002;3(7):1000-1004
2. Tateishi-Yuyama E, Matsubara H, Murohara T. et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002Aug10;360(9331):427-35
3. Ikenaga S, Hamano K, Nishida M. et al. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J Surg Res. 2001;96(2):277-283
4. Ji WF, Tong PJ, Zheng WB, Li J, Xiao LW. Experimental Study on Treatment of Femoral Head Necrosis with Arterial Perfusion of Marrow Stem Cells. Chin J Integr Trad West Med. 2004;24(11):999-1002
5. Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009Apr;27(4):442-6
6. Deng YB, Yuan QT, Liu XG. et al. Functional recovery after rhesus monkey spinal cord injury by transplantation of bone marrow mesenchymal-stem cell-derived neurons. Chin Med J (Engl). 2005;118(18):1533-1541
7. Lange C, Togel F, Ittrich H. et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68(4):1613-1617
8. Li ZH, Liao W, Cui XL. et al. Effects of Cbfa1 on osteoanagenesis during avascular necrosis of femoral head. Sci Res Essays. 2010;5(18):2721-2730
9. Zhao Y, Lu YL, Qiao Q, Du ZY, Xu YJ, Qi KM. Transfection of enhenced green fluorescent protein gene into mesenchymal stem cells and its expression. Chin J Exp Surg. 2004;21(1):24-25
10. Edinger M, Cao YA, Verneris MR. et al. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101(2):640
11. Annaloro C, Onida F, Lambertenghi Deliliers G. Autologous hematopoietic stem cell transplantation in autoimmune diseases. Expert Rev Hematol. 2009Dec;2(6):699-715
12. Rosato E, Pisarri S, Salsano F. Current strategies for the treatment of autoimmune diseases. J Biol Regul Homeost Agents. 2010;24(3):251-259
13. Karussis D, Karageorgiou C, Vaknin-Dembinsky A. et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010Oct;67(10):1187-1194
14. Szodoray P, Varoczy L, Szegedi G, Zeher M. Autologous stem cell transplantation in autoimmune and rheumatic diseases: from the molecular background to clinical applications. Scand J Rheumatol. 2010;39(1):1-11
15. Nagaya N, Fujii T, Iwase T. et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004Dec;287(6):H2670-2676
16. Mazo M, Planat-benard V, Abizanda G. et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10(5):454-462
17. Mazo M, Gavira JJ, Abizanda G. et al. Transplantation of mesenchymal stem cells exerts a greater long-term effect than bone marrow mononuclear cells in a chronic myocardial infarction model in rat. Cell Transplant. 2010;19(3):313-28
18. Mark AL, Sun Z, Warren DS. et al. Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg. 2010Oct;252(4):591-596
19. Shi XL, Gu JY, Han B. et al. Magnetically labeled mesenchymal stem cells after autologous transplantation into acutely injured liver. World J Gastroenterol. 2010Aug7;16(29):3674-3679
20. Walker PA, Shah SK, Jimenez F. et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010Oct;225(2):341-352
21. Zhang H, Liu Z, Li R. et al. Transplantation of embryonic small hepatocytes induces regeneration of injured liver in adult rat. Transplant Proc. 2009Nov;41(9):3887-3892
22. Lai KA, Shen WJ, Yang CY. et al. The use of alendronate to prevent early collapse of the femoral head in patientswith nontraumatic osteonecrosis. J Bone Joint Surg Am. 2005;87(10):2155-2159
23. Wang CJ, Wang FS, Huang CC. et al. Treatment for osteonecrosis of the femoral head: comparison of extra corporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg Am. 2005;87(11):2380-2387
24. Reis ND, Schwartz O, Militianu D. et al. Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br. 2003;85(3):371-375
25. Castro FP, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop. 2000;29:187-194
26. Shannon BD, Trousdale RT. Femoral osteotomies for avascular necrosis of the femoral head. Clin Orthop Relat Res. 2004(418):34-40
27. Nakamura Y, Kumazawa Y, Mitsui H. et al. Combined rotational osteotomy and vascularized iliac bone graft for advanced osteonecrosis of the femoral head. J Reconstr Microsurg. 2005;21(2):101-105
28. Wang BL, Sun W, Shi ZC. et al. Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop. 2010Jun;34(5):635-639
29. Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139-145
30. Mont MA, Seyler TM, Marker DR. et al. Use of metal-on-metal total hip resurfacing for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 2006;88(Suppl 3):90-97
31. Rijnen WH, Lameijn N, Schreurs BW. et al. Total hip arthroplasty after failed treatment for osteonecrosis of the femoral head. Orthop Clin North Am. 2009Apr;40(2):291-298
32. Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005Mar;87:106-12
33. Wang BL, Sun W, Shi ZC. et al. Treatment of nontraumatic osteonecrosis of the femoral head with the implantation of core decompression and concentrated autologous bone marrow containing mononuclear cells. Arch Orthop Trauma Surg. 2010Jul;130(7):859-865
34. Hernigou P, Poignard A, Zilber S. et al. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009Jan;43(1):40-45
35. Sun Y, Feng Y, Zhang C. The effect of bone marrow mononuclear cells on vascularization and bone regeneration in steroid-induced osteonecrosis of the femoral head. Joint Bone Spine. 2009Dec;76(6):685-690
36. Gangji V, Toungouz M, Hauzeur JP. Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. 2005Apr;5(4):437-442
37. Tauchmanovà L, De Rosa G, Serio B. et al. Avascular necrosis in long-term survivors after allogeneic or autologous stem cell transplantation: a single center experience and a review. Cancer. 2003;97(10):2453-2461
38. Lazarus HM, Haynesworth SE, Gerson SL. et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557-564
39. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815-1822
40. Liu L, Sun Z, Chen B. et al. Ex vivo expansion and in vivo infusion of bone marrow-derived Flk-1+CD31-CD34- mesenchymal stem cells: feasibility and safety from monkey to human. Stem Cells Dev. 2006;15(3):349-357
41. Devine SM, Bartholomew AM, Mahmud N. et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29(2):244-255
42. Liu LH, Sun QY, Hu KX. et al. Safety evaluation of allogenic rhesus mesenchymal stem cells infusion and detection of chimerism. J Sec Milit Med Univ. 2005;26(3):263-266
43. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007Nov;25(11):2739-49
44. Yagi H, Soto-Gutierrez A, Parekkadan B. et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19(6):667-79
45. Mouiseddine M, François S, Semont A. et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80:S49-55
46. François S, Bensidhoum M, Mouiseddine M. et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006Apr;24(4):1020-9
47. Adams GB, Chabner KT, Foxall RB. et al. Heterologous cells cooperate to augment stem cell migration, homing, and engraftment. Blood. 2003;101(1):45-51
48. Helmuth L. Stem cells hear call of injured tissue. Science. 2000;290(5496):1479-1481
Author contact
![]() Corresponding author: Zhang-hua Li, Tel: +8627-88041911-82209; Email: li1663net
Corresponding author: Zhang-hua Li, Tel: +8627-88041911-82209; Email: li1663net

 Global reach, higher impact
Global reach, higher impact