3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2010; 7(1):43-47. doi:10.7150/ijms.7.43 This issue Cite
Research Paper
Growth of Microorganisms in Total Parenteral Nutrition Solutions Without Lipid
1. Preclinical Assessment Department, Otsuka Pharmaceutical Factory, Inc., 115 Tateiwa, Naruto, Tokushima 772-8601, Japan.
2. Surgery, Kawasaki Hospital, 3-3-1 Higashiyamamachi, Hyougo-ku, Kobe 652-0042, Japan
Received 2009-10-13; Accepted 2010-1-21; Published 2010-1-22
Abstract
Background: To identify the microorganisms that can grow rapidly in total parenteral nutrition (TPN) solutions, we investigated the growth of the major causes of catheter-related blood stream infection (Staphylococcus aureus, Serratia marcescens, Bacillus cereus, and Candida albicans) in TPN solutions without lipid. Methods: Experiment 1: A commercial TPN solution without lipid containing multivitamins (pH5.6) was used. A specific number of each test microorganism was added to each 10 mL of the TPN solution and incubated at room temperature. An aliquot of test solution was sampled and inoculated to SCD agar plates at 0, 24, and 48 hrs after the addition of the microorganisms. The number of microorganisms was counted as colony forming units. Experiment 2: The other 2 commercial TPN solutions without lipid (pH5.5) were supplemented with multivitamins. The pH values of the solutions were adjusted to about 6.0, 6.5, or 7.0 using 0.5 mol/L NaOH. The addition of microorganisms, incubation, and counting were performed in the same manner. Results: Experiment 1: S. aureus, S. marcescens, and B. cereus did not increase in the TPN solution without lipid containing multivitamins (pH5.6), but C. albicans increased rapidly. Experiment 2: The 3 bacterial species did not increase even at pH6.0, but increased at pH6.5 and increased rapidly at pH7.0 in both TPN solutions. C. albicans increased similarly at any pH. Conclusion: These results suggest that bacterial species cannot grow in TPN solutions without lipid due to the acidity (pH5.6 or lower), but Candida species can grow regardless of the acidity.
Keywords: CRBSI, microbial growth, growth of microorganism, pH, TPN solution
INTRODUCTION
Catheter-related blood stream infection (CRBSI) is one of the most common complications of intravenous catheters.1-3 Total parenteral nutrition (TPN) solutions are considered to be relatively good growth mediums for microorganisms due to the components.3,4 To reduce or to prevent CRBSI, the growth properties of the microorganisms causing CRBSI in TPN solutions should be understood. Also, the factors which enhance or inhibit microbial growth should be investigated and identified. Because most TPN solutions used in Japan do not contain lipid, we first investigated the growth of the microorganisms in TPN solutions without lipid in this study. All TPN solutions used here contained multivitamins, because Staphylococcus aureus needs multivitamins (or lipid) to increase.5
As the major causes of CRBSI, Staphylococcus aureus, Staphylococcus epidermidis, Serratia marcescens, Escherichia coli, Klebsiella pneumonia, Candida albicans, etc. were shown.1,2,6,7 Furthermore, blood stream infection outbreaks of Bacillus cereus via intravenous line were recently reported in Japan.8 In the present study, therefore, Staphylococcus aureus as a delegate of gram positive cocci, Serratia marcescens as a delegate of gram negative rods, Bacillus cereus as a delegate of gram positive rods, and Candida albicans as a delegate of fungi were examined, and one standard strain and 2 or 3 clinical isolates of each species were employed. To identify which microorganism can grow in TPN solutions, the growth of all strains employed were examined in a commercial TPN solution without lipid in the 1st experiment. To identify which factor is effective to inhibit microbial growth, the growth of the standard strain of each species was examined at different pH values in 2 different osmotic pressure TPN solutions in the 2nd experiment.
MATERIALS AND METHODS
Microorganisms employed
Experiment 1: A standard American Type Culture Collection (ATCC) strain and 2 or 3 clinical isolates were used for each microorganisms; the standard strain ATCC6538 and 3 clinical isolates (N1, N2, N3) of Staphylococcus aureus, the standard strain ATCC13880 and 2 clinical isolates (N4, N5) of Serratia marcescens, the standard strain ATCC11778 and 3 clinical isolates (H1, H2, H3) of Bacillus cereus, and the standard strain ATCC10231 and 3 clinical isolates (N6, N7, N8) of Candida albicans.
Experiment 2: The standard strain was used for each microorganism; the ATCC653 of S. aureus, the ATCC13880 of S. marcescens, the ATCC11778 of B. cereus, and the ATCC10231 of C. albicans.
Test solutions
Experiment 1: A commercial TPN solution without lipid containing multivitamins (NP1; NEOPAREN-1, Otsuka Pharmaceutical Factory, Inc., Japan) was used. The composition of NP1 is shown in Table 1, and the pH value is 5.6 and the osmotic pressure ratio to physiological saline (OPR) is approximately 4.
Experiment 2: The other 2 commercial TPN solutions without lipid (AMINOTRIPA-1 and AMINOTRIPA-2, Otsuka Pharmaceutical Factory, Inc.) were supplemented with multivitamins (Otsuka MV Injection, Otsuka Pharmaceutical Factory, Inc.), and were used as the base solutions (AT1V and AT2V). The compositions of AT1V and AT2V are also shown in Table 1. The pH value and OPR of AT1V are 5.5 and approximately 5, and those of AT2V are 5.5 and approximately 6. The other test solutions were prepared by adjusting the pH of AT1V and AT2V to about 6.0, 6.5, or 7.0 using 0.5 mol/L NaOH. The pH values of the prepared solutions were measured with the pH meter (SevenEasy S20, Mettler-Toledo GmbH, Switzerland), and the results are shown in Table 2.
The compositions of NP1 AT1V and AT2V
| Solution | NP1 | AT1V | AT2V | |
|---|---|---|---|---|
| (volume) | (1000 mL) | (850 mL) | (900 mL) | |
| Amino acids | 30.0 g | 25.0 g | 30.0 g | |
| Glucose | 120 g | 79.8 g | 100.2 g | |
| Fructose | - | 40.2 g | 49.8 g | |
| Xylitol | - | 19.8 g | 25.2 g | |
| Na+ | 50 mEq | 35 mEq | 35 mEq | |
| K+ | 22 mEq | 22 mEq | 27 mEq | |
| Mg2+ | 4 mEq | 4 mEq | 5 mEq | |
| Ca2+ | 4 mEq | 4 mEq | 5 mEq | |
| Cl- | 50 mEq | 35 mEq | 35 mEq | |
| SO42- | 4 mEq | 4 mEq | 5 mEq | |
| Acetate- | 36 mEq | 44 mEq | 54 mEq | |
| Gluconate- | - | 4 mEq | 5 mEq | |
| Citrate3- | 4 mEq | 10 mEq | 11 mEq | |
| P | 156 mg | 154 mg | 186 mg | |
| Zn | 20 μmol | 8 μmol | 10 μmol | |
| Vitamin B1 | 1.53 mg | 3.1 mg | 3.1 mg | |
| Vitamin B2 | 1.8 mg | 3.6 mg | 3.6 mg | |
| Vitamin B6 | 2.02 mg | 4.0 mg | 4.0 mg | |
| Vitamin B12 | 0.0025 mg | 0.005 mg | 0.005 mg | |
| Vitamin C | 50 mg | 100 mg | 100 mg | |
| Folic acid | 0.2 mg | 0.4 mg | 0.4 mg | |
| Nicotinamide | 20 mg | 40 mg | 40 mg | |
| Biotin | 0.03 mg | 0.06 mg | 0.06 mg | |
| Panthenol | 7 mg | 14 mg | 14 mg | |
| Vitamin A | 1650 IU | 3300 IU | 3300 IU | |
| Vitamin D3 | 0.0025 mg | 0.005 mg | 0.005 mg | |
| Vitamin E | 5 mg | 10 mg | 10 mg | |
| Vitamin K | 1 mg | 2 mg | 2 mg | |
| pH | 5.6 | 5.5 | 5.5 | |
| Osmotic pressure ratio to physiological saline | Approx. 4 | Approx. 5 | Approx. 6 | |
pH values of test solutions
| Test solution | Target pH | Measured pH |
|---|---|---|
| AT1V | - | 5.48 (original pH) |
| 6.0 | 5.99 | |
| 6.5 | 6.44 | |
| 7.0 | 6.87 | |
| AT2V | - | 5.54 (original pH) |
| 6.0 | 5.95 | |
| 6.5 | 6.42 | |
| 7.0 | 6.82 |
Addition of microorganism, incubation and sampling
A specific number of each test microorganism was added to each 10 mL of test solutions in sterile plastic tubes, and all tubes were allowed to stand at room temperature (24-27ºC). An aliquot of test solution was sampled at 0, 24, and 48 hours after the addition of microorganisms.
Measurement of viable microorganisms
Each aliquot sampled was inoculated in Soybean Casein Digest (SCD) agar plate in duplicate. When necessary, the aliquot was diluted 10-fold to 107-fold with physiological saline before inoculating. After 24-hour incubation at 37ºC, the number of colony forming units (CFU) of each microorganism was counted for each plate. The mean CFU of duplicate data was calculated for each aliquot, and the number of each microorganism per mL was calculated using the number of CFU per plate, aliquot volume, and diluting ratio. The results are shown as the values of CFU/mL in semi-logarithmic graphs.
The data from 1 standard strain and 2 or 3 clinical isolate strains of each species at 24 and 48 hours (Experiment 1) were analyzed using Student's t-test against the initial data (at 0 hour) to confirm the generality of the results for each species.
RESULTS
Experiment 1
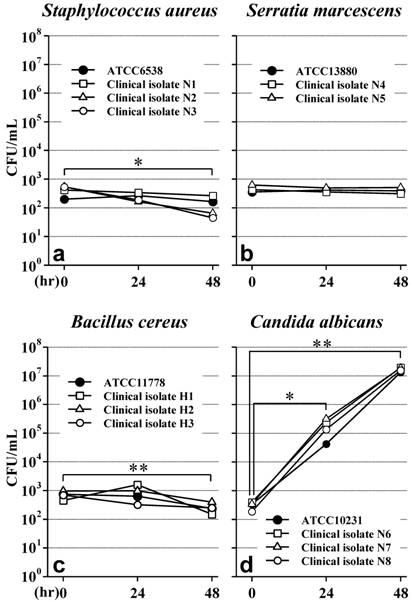
All 4 strains of S. aureus did not increase in NP1 for 48 hours, and isolates N2 and N3 seemed to decrease (Figure 1a). There was a significant decrease (p<0.05) in the mean value of the 4 strains at 48 hours after the addition of microorganisms.
All 3 strains of S. marcescens did not increase or decrease in NP1 for 48 hours (Figure 1b), and there was no significant difference in the mean values of the 3 strains.
All 4 strains of B. cereus did not increase in NP1 for 48 hours (Figure 1c), and there was a significant decrease (p<0.01) in the mean value of the 4 strains at 48 hours.
On the other hand, all 4 strains of C. albicans increased rapidly, reaching the maximum concentration within 48 hours (Figure 1d). There were significant increases in the mean values of the 4 strains both at 24hours (p<0.05) and at 48 hours (p<0.01).
Experiment 2
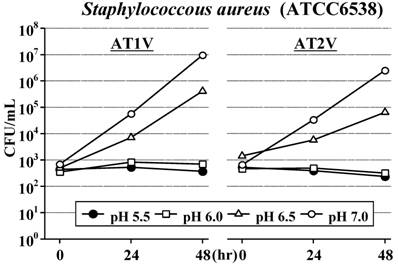
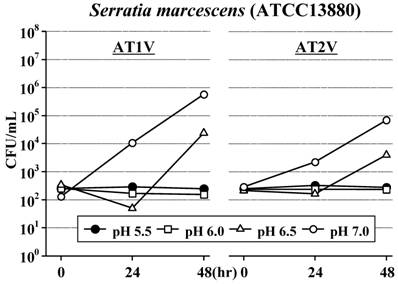
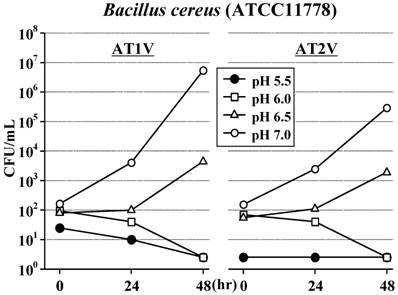
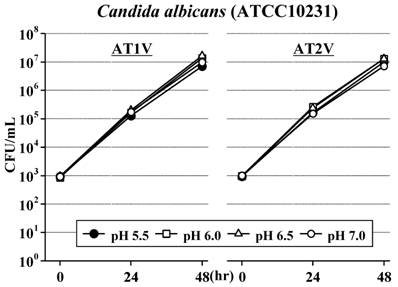
In AT1V, the standard strain of S. aureus did not increase for 48 hours at pH6.0 as well as at the original pH5.5, but increased at pH6.5 and increased rapidly at pH7.0 (Figure 2). The standard strains of S. marcescens (Figure 3) and B. cereus (Figure 4) also did not increase for 48 hours at pH5.5 and pH6.0; furthermore, these strains did not increase even at pH6.5 for 24 hours. However, these strains increased at pH7.0 as S. aureus. On the other hand, the standard strain of C. albicans increased rapidly and equally at any pH value, and reached the maximum concentration within 48 hours (Figure 5).
Also in AT2V, the standard strains of S. aureus (Figure 2), S. marcescens (Figure 3) and B. cereus (Figure 4) did not increase at pH5.5 and pH6.0, but increased at pH6.5 and pH7.0, except that the growing rates were somewhat less than those in AT1V. The standard strain of C. albicans increased rapidly and reached the maximum concentration within 48 hours at any pH values in AT2V, and the growing rates were the same as those in AT1V (Figure 5).
Growth of Staphylococcus aureus (a) Serratia marcescens (b), Bacillus cereus (c) and Candida albicans (d) in NP1 (pH5.6, OPR is approximately 4). The data from 3 or 4 strains of each species at 24 and 48 hrs were analyzed using Student's t-test against the initial data (at 0 hr). *, p<0.05 vs 0hr. **, p<0.01 vs 0 hr. OPR, osmotic pressure ratio to physiological saline.
Effect of pH on the growth of Staphylococcus aureus in AT1V (pH55, OPR is approximately 5) and AT2V (pH5.5, OPR is approximately 6). The pH value was adjusted by adding 0.5 mol/L NaOH. OPR, osmotic pressure ratio to physiological saline.
Effect of pH on the growth of Serratia marcescens in AT1V (pH55, OPR is approximately 5) and AT2V (pH5.5, OPR is approximately 6). The pH value was adjusted by adding 0.5 mol/L NaOH. OPR, osmotic pressure ratio to physiological saline.
Effect of pH on the growth of Bacillus cereus in AT1V (pH55, OPR is approximately 5) and AT2V (pH5.5, OPR is approximately 6). The pH value was adjusted by adding 0.5 mol/L NaOH. OPR, osmotic pressure ratio to physiological saline.
Effect of pH on the growth of Candida albicans in AT1V (pH55, OPR is approximately 5) and AT2V (pH5.5, OPR is approximately 6). The pH value was adjusted by adding 0.5 mol/L NaOH. OPR, osmotic pressure ratio to physiological saline.
DISCUSSION
To reduce catheter-related blood stream infection (CRBSI), we have to understand the growth properties of the microorganisms causing CRBSI. Therefore, we investigated the growth of the microorganisms that are known as the major causes of CRBSI, Staphylococcus aureus, Serratia marcescens, Bacillus cereus and Candida albicans, in total parenteral nutrition (TPN) solutions without lipid.
In a commercial TPN solution without lipid containing multivitamins (NP1; the pH value is 5.6 and the osmotic pressure ratio to physiological saline [OPR] is approximately 4), all of the standard strains and the clinical isolates of S. aureus, S. marcescens, and B. cereus did not increase. On the other hand, all strains of C. albicans increased rapidly in NP1. These results of Experiment 1 suggested that bacterial species such as Staphylococcus, Serratia and Bacillus can not increase in acidic (pH5.6 or lower) TPN solutions. In fact, the standard strains of these 3 bacterial species did not increase in the other TPN solutions (AT1V; the pH value is 5.5 and the OPR is approximately 5. AT2V; the pH value is 5.5 and the OPR is approximately 6). These standard strains of bacterial species did not increase even at pH6.0, but increased at pH6.5 and pH7.0. At pH6.5 and pH7.0, the growth rates of the standard strains of S. aureus, S. marcescens and B. cereus seemed to be suppressed slightly in AT2V (the OPR is approximately 6) compared with AT1V (the OPR is approximately 5). However, the standard strain of C. albicans increased similarly at any pH and both in AT1V and AT2V as well as in NP1.
TPN solutions are considered to be relatively good growth mediums for microorganisms due to the components,3,4 whereas a number of investigators have shown that TPN solutions containing hypertonic glucose and amino acids are poor growth media for most nosocomial pathogens, with the exception of Candida and other yeasts.6,9-11 They estimated that the acidic pH and/or the hyperosmolality might suppress the bacterial growth.9,10,12 The results of 3 bacterial species in this study demonstrated that the acidity of the TPN solution is the critical factor suppressing the bacterial growth, but that the hyperosmolality of conventional TPN solutions has a little effect. On the other hand, the results of C. albicans in this study demonstrated that both the acidic pH and the hyperosmolality of TPN solutions hardly have an effect on Candida growth as reported in other studies.6,11,12
These results suggest that bacterial species cannot grow in TPN solutions without lipid due to the acidity (pH5.6 or lower), but Candida species can grow regardless of the acidity.
Acknowledgements
We are very grateful to Mr. Masao Ichihara for his helpful comments and to Mr. Takumi Tamura for his expert technical assistance.
CONFLICT OF INTERESTS
The authors have declared that no conflict of interest exists.
References
1. Mermel LA, Farr BM, Sherertz RJ. et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249-1272
2. Llop J, Badia MB, Comas D, Tubau M, Jodar R. Colonization and bacteremia risk factors in parenteral nutrition catheterization. Clin Nutr. 2001;20:527-534
3. Banton J. Techniques to prevent central venous catheter infection: products, research, and recommendations. Nutr Clin Pract. 2006;21:56-61
4. Allwood MC. Microbiological risks in parenteral nutrition compounding. Nutrition. 1997;13:60-61
5. Shimono K, Kaneda S, Kuwahara T, Kawaguchi Y, Momii A. Effects of lipid and multivitamins on the growth of Staphylococcus aureus in peripheral parenteral nutrition solutions. Clin Nutr. 2005;24:706-707
6. Rowe CE, Fukuyama TT, Martinoff JT. Growth of microorganisms in total nutrient admixtures. Drug Intell Clin Pharm. 1987;21:633-638
7. Crocker KS, Noga R, Filibeck DJ, Krey NH, Markovic M, Steffee WP. Microbial growth comparisons of five commercial parenteral lipid emulsions. J Parent Enter Nutr. 1984;8:391-395
8. Matsumoto S, Suenaga H, Naito K, Sawazaki M, Hiramatsu T, Agata N. Management of suspected nosocomial infection: an audit of 19 hospitalized patients with septicemia caused by Bacillus species. Jpn J Infect Dis. 2000;53:196-202
9. Didier ME, Fischer S, Maki DG. Total nutrient admixtures appear safer than lipid emulsion alone as regards microbial contamination: growth properties of microbial pathogens at room temperature. J Parent Enter Nutr. 1998;22:291-296
10. Gilbert M, Gallagher SC, Eads M, Elmore MF. Microbial growth patterns in a total parenteral nutrition formulation containing lipid emulsion. J Parent Enter Nutr. 1986;10:494-497
11. Melly MA, Meng HC, Schaffner W. Microbial growth in lipid emulsions used in parenteral nutrition. Arch Surg. 1975;110:1479-1481
12. Holmes CJ, Allwood MC. The growth of micro-organisms in parenteral nutrition solutions containing amino acids and sugars. Int J Pharmaceu. 1979;2:325-335
Author contact
![]() Corresponding author: Takashi Kuwahara, Ph.D., Preclinical Assessment Department, Otsuka Pharmaceutical Factory, Inc., 115 Tateiwa, Naruto, Tokushima 772-8601, Japan. Telephone: +81 88 685 1151 (Ext. 678); Fax: +81 88 684 0553; E-mail: kuwahat2co.jp
Corresponding author: Takashi Kuwahara, Ph.D., Preclinical Assessment Department, Otsuka Pharmaceutical Factory, Inc., 115 Tateiwa, Naruto, Tokushima 772-8601, Japan. Telephone: +81 88 685 1151 (Ext. 678); Fax: +81 88 684 0553; E-mail: kuwahat2co.jp

 Global reach, higher impact
Global reach, higher impact