3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(5):258-264. doi:10.7150/ijms.6.258 This issue Cite
Research Paper
Treatment of proximal femur infections with antibiotic-loaded cement spacers
1. Klinik für Orthopädie und Orthopädische Chirurgie, Universitätskliniken des Saarlandes, Homburg/Saar, Germany
2. Chirurgisch-Orthopädisches Zentrum Illingen/Saar, Germany
3. Amper Kliniken AG, Klinikum Dachau, Abteilung für Orthopädie und Unfallchirurgie, Germany
Received 2009-8-1; Accepted 2009-9-2; Published 2009-9-3
Abstract
In case of periprosthetic hip infections the implantation of antibiotic-loaded PMMA spacers is accepted for an adequate treatment option. Although their indication for the treatment of destructive, bacterial infections of the proximal femur would make sense, literature data are scarce. Hence, the aim of this study was to evaluate the efficacy of antibiotic-impregnated spacers in the treatment of proximal femur infections.
In 10 consecutive patients (5 M/ 5 F, mean age 66 y.) with bacterial proximal femur infections, a femoral head/neck resection was prospectively performed with a subsequent implantation of an antibiotic-loaded spacer. The joint-specific outcome was evaluated by the Merle d´Aubigne and the Mayo hip score, the general outcome by SF-36. The time periods were divided into “infection situation”, “between stages” and meanly 1 year “after prosthesis implantation”.
The spacers were meanly implanted over 90 [155-744] days. In all cases an infection eradication could be achieved. After infection eradication, a prosthesis implantation was performed in 8 cases. The general scores showed significant increases at each time period. With regard to the dimension “pain”, both scores demonstrated a significant increase between “infection situation” and “between stages”, but no significance between “between stages” and “after prosthesis implantation”.
Spacers could be indicated in the treatment of proximal femur infections. Besides an infection eradication, a pain reduction is also possible.
Keywords: hip spacer, proximal femur infection, hip joint, antibiotic-loaded cement
Introduction
The maintenance of the joint function and the infection eradication are the treatment aims of bacterial infections of the proximal femur and its bordering soft tissues. In case of early infections of a bacterial coxitis, local treatment procedures, such as arthrotomy and lavage [2], open or arthroscopic joint lavage [4], insertion of antibiotic-loaded media [21] and systemic antibiosis [2] usually lead to a successful infection management. However, these procedures are insufficient in the treatment of the destructive, bacterial coxitis or the septic pseudarthrosis of the femoral neck after osteosynthesis. Thus, in these cases a two-stage treatment is often required. Beyond the obligate systemic antibiosis, the common procedure includes an excision arthroplasty of the femoral head (Girdlestone-hip) with a simultaneous insertion of commercial antibiotic-loaded device (beads or collagen sponges) [16-18, 20]. In case of multimicrobial infections, these commercial antibiotic-impregnated media cannot provide frequently a sufficient antibiotic therapy. Further disadvantages of the Girdlestone-hip are the instable joint situation and the soft-tissue shortening which may lead to enormous problems during the later prosthesis reimplantation [5, 14, 24].
A modern, innovative procedure for avoidance of soft-tissue shortening and provision of sufficient infection therapy is the usage of temporary, antibiotic-loaded cement spacers [5, 7, 14, 24]. Although their indication in the treatment of destructive, bacterial infections of the proximal femur would make sense, literature data are scarce [8-9].
In this study, we report on the technical procedure and the outcome of our therapy concept using antibiotic-impregnated PMMA hip spacers in the treatment of proximal femur infections.
Patients and Methods
Patients
Between 2000 and 2004 we performed an excision arthroplasty of the femoral head/neck in 10 consecutive patients (5 M, 5 F) due to bacterial infections of the proximal femur. A total of 11 antibiotic-loaded PMMA hip spacers were implanted (Table 1). At the time of surgery, the mean age of the patients was 66 [52-77] years. After infection eradication, a prosthesis has been reimplanted in 8 cases. One patient passed away due to an unclear cause between stages, another patient (bilateral spacer implantation) due to a cardiomyopathy. In both cases, a reinfection could be excluded by magnet resonance imaging (MRI).
Patients' comorbidities, surgical procedures, pathogen organisms, time between stages and follow-up are summarized in Table 1. The diagnostic criteria for infection consisted of medical history, physical examination, blood results, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), radiological findings (x-ray, CT or MRI) and isolation of the pathogen organism. In 2 cases, no organism could be identified, however, the histopathological findings confirmed the diagnosis of an osteomyelitis of the femoral head.
Patients' data, surgical procedures, and causative organisms at the site of hip spacer implantation in the treatment of coxitis and proximal femur infections after osteosynthesis.
| Patient | Age/ Gender | Diagnosis | Surgical treatment | Pathogen organism | Time between stages [days] | Follow-up [days] | Comorbidities |
|---|---|---|---|---|---|---|---|
| 1 | 61/M | reactive coxitis after psoas abscess | femoral head resection and spacer implantation | n.o.i. | 84 | 684 | cerebral infarct, renal tuberculosis, heart muscle akinesia |
| 2 | 65/F | septic pseudarthrosis after osteosynthesis for intertrochanteric fracture | dynamic hip screw removal, femoral head resection and spacer implantation | MRSA S. epidermidis | 87 | 473 | hyperthyreosis |
| 3 | 52/M | destructive bacterial coxitis | resection arthroplasty, beads implantation and subsequent spacer implantation | S. aureus | 60 | 405 | arterial hypertension, hyperuricaemia, obesity, diabetes mellitus |
| 4 | 66/F | secondary bacterial coxitis after pelvic abscess | femoral head resection and spacer implantation | S. aureus | 93 | 744 | arterial hypertension, alcohol abuse, polyneuropathia |
| 5 | 66/M | septic pseudarthrosis after osteosynthesis for intertrochanteric fracture | hardware removal, femoral head resection and spacer spacer implantation | α-haemol. streptococci | 192 | 175 | adrenal adenoma, arterial hypertension, diabetes mellitus, peripheral vascular disease, heart insufficiency NYHA II, obstructive pulmonal disease |
| 6 | 75/F | septic pseudarthrosis after osteosynthesis for intertrochanteric fracture | dynamic hip screw removal, femoral head resection and spacer implantation | n.o.i. | 73 | 210 | heart infarct, chronic venous stasis, gastric ulcer |
| 7 | 77/M | septic pseudarthrosis after osteosynthesis for intertrochanteric fracture | dynamic hip screw removal, femoral head resection and spacer implantation | S. aureus | 134 | 344 | arterial hypertension, alcohol abuse, chronic renal insufficiency, coronar heart disease, cerebral atrophy |
| 8 | 70/F | destructive bacterial coxitis | femoral head resection and spacer implantation | S. aureus | 113 | 155 | obesity, arterial hypertension, reflux oesophagitis, local hypernephroma relapse |
| 9 | 72/M | bilateral destructive bacterial coxitis following bilateral psoas abscess | bilateral abscess debridement, femoral head resection and spacer implantation | S. aureus | p.p.a. | p.p.a. | lunge edema, hemicolectomy, sepsis |
| 10 | 52/F | destructive bacterial coxitis | femoral head resection and spacer implantation | n.o.i. | p.p.a. | p.p.a. | arterial hypertension, heart insufficiency, depression, spondylodiscitis L5/S1 |
n.o.i.: no organism identified; p.p.a.: patient passed away
Methods
Surgical approach for spacer implantation
Via a transgluteal approach the proximal femur was demonstrated. After radical debridement of potentially infected and necrotic soft-tissues, the femoral head was resected under consideration of the later implantation of the prosthesis into the proximal femur. Tissue samples (bone- and soft tissue) were sent for bacteriological and histological examination. After proper leg positioning, the femur was prepared with the rasps of our endoprosthesis systems (Bicontact®, Fa. Aesculap, Tuttlingen, Germany) for the spacer implantation. Afterwards, pulsatile lavage was performed with approximately 15 l Ringer's solution PL 2511 (Fa. Fresenius-Kabi, Bad-Homburg, Germany).
At the same time, another team in the surgery room had been producing the spacer by using a CAD-planned and CNC-milled, two-parted mould of polyoxymethylene [1]. The bone cement used in all cases was Refobacin-Palacos ® (Fa. Merck, Darmstadt, Gemany), each spacer was loaded with 4 g vancomycin (Fa. cell pharen GmbH, Hannover, Germany) per 80 g cement. In one case, 800 mg teicoplanin were used due to a vancomycin allergy of the patient.
All spacers have been fixed to the proximal femur according either to the “glove”-technique [1] or to a “press-fit”-method. Thus, a rotation-secure implantation could be achieved in the proximal marrow cavity of the femur. After spacer reduction, a redon drain was placed at the spacer's head and another one subfascial. The wound was then closed in layers.
Postoperative treatment
Antibiosis
After consultation with our Microbiologic Institute and under narrow CRP monitoring, intravenous antibiotics have been administered for the first 4 weeks and subsequently oral antibiotics for another two weeks, depending on the sensitivity profile of the particular causative organism. Both patients with no isolated organisms were treated with flucloxacillin and clindamycin, respectively. The systemic therapy was ended if the CRP level was normal after these 6 weeks. 14 days after ending of the antibiosis and if the CRP has returned to normal levels, the prosthesis implantation could be planned.
Physiotherapy
Postoperatively, an immediate mobilisation of the patients with crutches under contact weight bearing (spacer not stable under total weight bearing) was aimed. The desired mobility of the operated hip joint should conform to the one of a hip joint with a standard prosthesis.
Surgical approach for prosthesis implantation
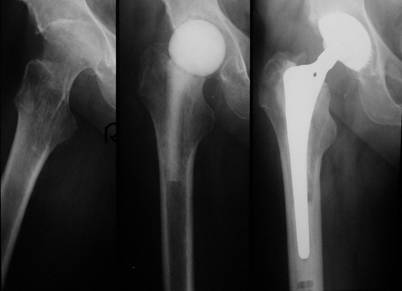
After demonstration of the spacer via the transgluteal approach, spacer removal, debridement and pulsatile lavage, we could implant a standard prosthesis type Aesculap Bicontact with a screw cup type SC (Fa. Aesculap, Tuttlingen, Germany) in 7 cases (Fig. 1). In one case a Link-revision stem (Fa. Waldemar Link, Hamburg, Germany) was implanted, whereas the acetabular cup was also a screw cup SC.
Left: Destructive bacterial coxitis; Middle: Spacer implantation between stages; Right: 3 months later and after infection eradication, a prosthesis implantation (SC® cup, Bicontact® stem, Fa. Aesculap, Tuttlingen, Germany) has been performed.
Follow-up after prosthesis implantation
Physical examination
Besides mobility and leg length measurement, the maximal walking distance, pain persistence and requirement for walking aids were evaluated.
Scores
Joint specific outcome
The joint specific outcome of the patients was evaluated by the Merle d´Aubigne [15] and Mayo Hip Score [10]. The selected time periods were “infection situation” (before the spacer implantation), “between stages” (after infection eradication, period between stages)) and “after prosthesis implantation”, at a mean follow-up of 1 year [155/744 days].
General outcome
The outcome of the patients was exclusively evaluated at the follow-up by the SF-36 [3], a questionnaire about the health related life quality. The evaluated scores of the patients were compared to ones of a control group of similar age and gender, representative of the german population.
Statistics
Due to the small sample size and the non-symmetrical distribution, the median and both extreme values are shown. Statistical analysis was performed with the Wilcoxon-test [28], significance niveau was defined for a p < 0.05. All statistical evaluation was carried out with the software program SPSS 12.0 (Fa. SPSS GmbH, Munich, Germany).
Results
Only the results of the eight patients with a prosthesis reimplantation have been evaluated. In all cases an infection eradication could be achieved. The spacers were meanly implanted for 90 [60/192] days.
1. Complications
A spacer dislocation occurred in one case. Treatment consisted of closed reduction and immobilization in a Newport orthesis (Fa. Ormed, Freiburg, Germany). The dislocation cause was a fracture of the dorsal acetabular lip which occurred during the femoral head dislocation. During stages, the patient suffered from a thrombosis, probably due to the tightness of the orthesis. One year later, we diagnosed in the same patient a septic prosthesis loosening again. The infection treatment consisted again of a spacer implantation. After infection eradication, a prosthesis was reimplanted. At a further follow-up of 24 months, no reinfection or infection persistence occurred.
2. Follow-up (meanly 1 year after prosthesis reimplantation [155/744 days])
2.1 Physical examination
Maximal walking distance
4 patients reported an unlimited walking distance, 2 patients were mobile only in their homes. One patient reported a walking distance of 200 m, however, he was dependent on a walking aid. One patient reported a weather-dependent insecurity beyond a distance of 200 m.
Pain
5 patients were painfree, one patient had moderate complaints after long walks. The other two patients reported of minor pain during mobilisation with crutches.
Walking aid
3 patients did not need any aid at all, one patient used an aid outdoors. One patient was dependent on an aid all the time due to a gluteal insufficiency. The other three patients were immobile during the implantation period and showed only minimal mobility with a walking frame.
Leg length discrepancy
At follow-up, a leg length discrepancy between 1 and 2.5 cm could be noticed in 3 patients, whereby in 2 out of the 3 cases this discrepancy has been decreased compared with the values before the spacer implantation, respectively.
3. Scores
3.1. Joint specific outcome
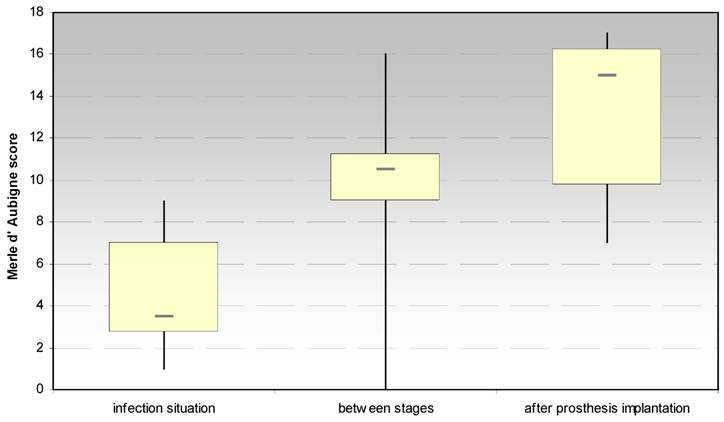
3.1.1 Merle d´Aubigné and Postel hip score (Fig. 2)
The evaluation of the Merle d´Aubigné and Postel hip score showed significant increases between the infection situation and the period between stages (p < 0.021) and the prosthesis reimplantation (p < 0.018), respectively. In regard to the score dimension “pain”, a significant increase (p < 0.018) between the infection situation and the period between stages could be achieved, but not to the prosthesis implantation.
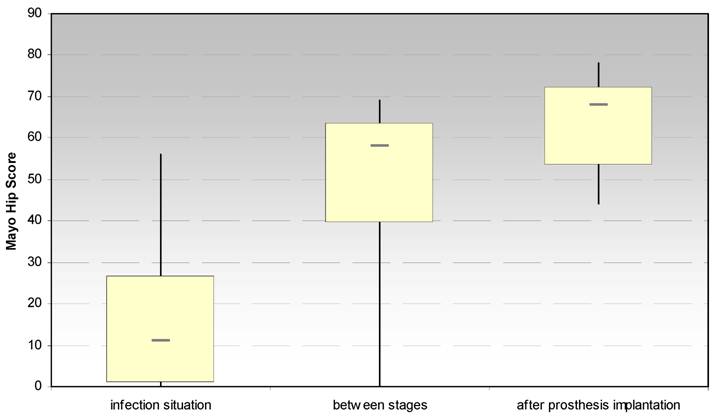
3.1.2. Mayo hip score after Kavanagh und Fitzgerald (Fig. 3)
The evaluation of the Mayo hip score showed also a significant increase between the infection situation and the period between stages (p < 0.028) and the prosthesis reimplantation (p < 0.018), respectively. Moreover, a significant increase (p < 0.026) has been noticed for the dimension “pain” after spacer implantation.
Evaluation of the hip joint function by the Merle d' Aubigne score at the site of spacer implantation in the treatment of proximal femur infections.
Evaluation of the hip joint function by the Mayo Hip Score at the site of spacer implantation in the treatment of proximal femur infections.
3.2. General outcome
3.2.1 SF-36
In the areas „ physical fitness“ and “physical role function“ the achieved values were below those of the control group. Regarding “pain”, “general health condition”, “social integration”, “emotional role function” and “mental well-being” they were beyond of those of the control group. Significant statistical test series could not be performed due to the small number of patients.
Discussion
The implantation of temporary, antibiotic-loaded PMMA prostheses is accepted for an adequate option in the treatment of periprosthetic infections. Although their indication for the treatment of destructive, bacterial infections of the proximal femur would make sense, literature data are scarce [8-9]. Thus, the aim of this article was to study the efficacy of antibiotic-loaded PMMA-hip spacers in the treatment of infections of the proximal femur.
Isiklar and colleagues were the first to report on the successful use of a hip spacer in the treatment of an infected femoral neck fracture with implant failure and pseudarthrosis [9]. Hsieh et al. treated 27 patients with deep hip infections following failed primary treatment of an intertrochanteric fracture with a two-stage protocol [8]. In the first 15 cases antibiotic-loaded beads have been implanted after resection arthroplasty, whereas the remaining 12 patients have been treated by implantation of an antibiotic-impregnated hip spacer. At an average follow-up of 4.8 years one reinfection could be observed in one patient in the first group. During the interim period, patients with a spacer prosthesis has significantly higher hip scores and better mobility after evaluation by the Merle d´Aubigné and Postel hip score. Similar to these data, we could not observe any reinfection or infection persistence in our patients' series.
The Girdlestone-hip (excision arthroplasty of the femoral head) with the subsequent insertion of local antibiotic-impregnated media is still counting among the standard treatment options of the destructive, bacterial coxitis [16-18, 20]. It is also performed in the treatment of the septic femoral neck pseudarthrosis. Frequently, pathogen organisms as tuberculosis and salmonella bacteria can be isolated from such infections [6, 13, 18, 20, 23]. With regard to these organisms and the increasing ratio of multiresistant bacteria [11-12, 25] a local antibiosis has become difficult to apply. Especially the ratio of multiresistant bacteria strains, as staphylococci, streptococci and enterococci, has increased [25-27]. These organisms were responsible for all infections in our patients. Commercially available antibiotic-loaded media (beads, collagen sponges) are loaded only with gentamicin. Therefore, the addition of an antibiotic to PMMA is required for enhancement of the antibiotic therapy which is possible using our treatment option.
To our knowledge, there exists only one study which compared the Girdlestone procedure with the spacer implantation with regard to the clinical outcome, surgical parameters and follow-up [7]. Although no significant difference could be observed regarding the infection eradication rate, many authors are in favour of the spacer procedure at the site of a two-stage protocol [5, 14, 24]. Especially the physiotherapeutical measurements can be performed better due to the spacer-induced joint stability [24]. The lacking leg length discrepany allows an almost physiological joint mobility which could serve in the prophylaxis of pneumonia and thrombosis [22]. Furthermore, some authors permit a partial weight bearing with the spacer [5, 19, 24], which can be performed painfree in most cases, in contrast to the excision arthroplasty [16]. A disuse osteoporosis and muscle atrophy are hereby prevented so that the later prosthesis reimplantation is facilitated [29]. In conclusion, the spacer implantation optimizes the premises for a successful reimplantation of the prosthesis with regard to the heart and circulation situation and the biomechanical properties.
Despite these advantages, no significant increase could be observed in our collective for the score dimension “mobility” between stages. On the contrary, the score values at follow-up showed an increase compared to pre- and during spacer implantation. A probable cause might be the reduced weight bearing properties of our spacer. Therefore, the enhancement of the spacer's stability should be the aim of further investigations, either with the insertion of a metallic endoskeleton or with K-wires [7-8, 14, 19].
Regarding the score dimension “pain” our patients showed significantly better results after the spacer implantation than before. Hereby, the articulating grinding of the spacer´s head against the acetabulum seems to be of no disadvantage. The pain reduction might result from the intra-articular pressure decrease due to the arthrotomy and head resection or the joint stability guaranteed by the spacer.
The evaluation of the follow-up results of the remaining parameters (walking aid, walking distance, joint mobility) showed satisfactory results. In only one case we could observe an unsatisfactory outcome. In particular, the consecutive complications (fracture of the dorsal acetabular lip, spacer dislocation, thrombosis, reinfection) had a negative influence on the outcome. The reinfection after the prosthesis reimplantation should not be attributed as a failure of the spacer treatment, because the reinfection rate after Girdlestone arthroplasty with 16.1 % [30] is higher than in our series.
The evaluation of the health-related life quality by the SF-36 showed that the values of the physical fitness and the physical role function were lower than those of the control group. These two scales reflect the health condition for normal and exhausting physical activity. In the scales “physical pain”, “general health perception”, “vitality”, “social integration”, “emotional role function” and “mental well-being” the values achieved were among the norm values. With regard to the health condition of the patients, our results indicate that the physical activity is affected after several operations. However, these affections do not have any severe influence on the normal social activity or create any emotional problems.
Conclusion
Spacers could be indicated in the treatment of proximal femur infections. Beyond the infection eradication a pain reduction is possible due to the spacer implantation. The mobility of the patients between stages could be enhanced by improving the spacer's mechanical properties.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Anagnostakos K, Köhler D, Schmitt E, Kelm J. The „glove“ - technique: a modified method for femoral fixation of antibiotic-loaded hip spacers. Acta Orthop. 2009 [epub ahead of print]
2. Broeng L, Hansen LB. Purulent coxitis after blockade treatment. Ugeskr Laeger. 1991;153:2433-2434
3. Bullinger M, Kirchberger I. Der SF-36-Fragebogen zum Gesundheitszustand: Handbuch für die deutschsprachige Fragebogenversion. Göttingen: Hogrefe Verlag. 1997
4. Carls J, Kohn D. Arthroskopische Therapie der eitrigen Coxitis. Arthroskopie. 1996;9:274-277
5. Deshmukh RG, Thevarajan K, Kok CS, Sivapathasundaram N, George SVN. An intramedullary cement spacer in total hip arthroplasty. J Arthroplasty. 1998;13:197-199
6. Gob A. Surgical treatment of coxitis tuberculosa. Z Orthop. 1980;118:55-60
7. Hsieh P-H, Shin C-H, Chang Y-H, Lee MS, Shin H-N, Yang W-E. Two stage Revision Hip Arthroplasty for Infection: Comparison between the Interim Use of Antibiotic-Loaded Cement Beads and a Spacer Prosthesis. J Bone Joint Surg. 2004;86:1989-1997
8. Hsieh P-H, Chang YH, Chen S-H, Shih C-H. Staged arthroplasty as salvage procedure for deep hip infection following intertrochanteric fracture. Int Orthop. 2006;30:228-32
9. Isiklar ZU, Demirors H, Akpinar S, Tandogan RN, Alparslan M. Two-stage treatment of chronic staphylocccal orthopaedic implant-related infections using vancomycin impregnated PMMA spacer and rifampin containing antibiotic protocol. Bull Hosp Jt Dis. 1999;58:79-85
10. Kavanagh BF, Fitzgerald RH. Clinical and roentgenographic assesssment of total hip arthroplasty. A new hip score. Clin Orthop. 1985;193:133-140
11. Kresken M, Hafner D, Witte W, Reinert RR. Resistenzentwicklung bei Staphylokokken und anderen grampositiven Erregern gegenüber Chemotherapeutika im mitteleuropäischen Raum. Chemother J. 2000;19:5-14
12. Kuechle DK, Landon GC, Musher DM, Noble PS. Elution of Vancomycin, Daptomycin and Amikacin from acrylic bone cement. Clin Orthop. 1991;264:302-308
13. Lavrov VN, Shchapov AIu, Tsoktoev DB. Endoprosthesis of the hip joint in progressive and chronic destructive tuberculous arthtitis: problems and prospects. Probl Tuberk. 2003;7:37-39
14. Leunig M, Chosa E, Speck M, Ganz R. A cement spacer for two-stage revision of infected implants of the hip joint. International Orthopaedics. 1998;22:209-214
15. Merle d Áubigne R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg (Am). 1954;36:451
16. Motzenbäcker C. Zur klinischen Problematik der Resektionshüfte untersucht am Krankengut der Orthopädischen Universitätsklinik Homburg/Saar der Jahre 1968-1987. Inauguraldissertation 1989. Saarlandes: Universität des Saarlandes. 1989
17. Müller KH. The therapy for pyogenic coxitis and its stabilisation with the fixateur externe (tubular system). Arch Orthop Trauma Surg. 1978;91:201-213
18. Sarkar MR, Rose C, Wachter N, Mohr W, Kinzl L. Bacterial coxitix caused by Salmonella enteridis. Case report and differential diagnostic considerations. Unfallchirurg. 1999;102:967-971
19. Schoellner C, Fuerderer S, Rompe JD, Eckardt A. Individual bone cement spacers (IBCS) for septic hip revision - preliminary report. Arch Orthop Trauma Surg. 2003;123:254-9
20. Schröder J, Palkovic S, Kipp F, Wassmann H. Salmonella enteritidis causing brain abscess and coxitis following intracranial surgery. Acta Neurochir. 2003;145:919-921
21. Schuckmann P, Schuckmann W. Personal experiences with the applications of septopal chains in the treatment of bacterial arthritis and osteomyelitis. Beitr Orthop Traumatol. 1989;36:428-434
22. Siebel T, Kelm J, Porsch M, Neumann WH, Regitz T. Two-Stage exchange of infected knee arthroplasty with an prosthesis-like interim cement spacer. Acta Orthop Belg. 2002;68:150-156
23. Sozio A, Paolemili D, Ciniglio FV. Compression arthrodesis in tuberculous coxitis. Arch Putti Chir Organi Mov. 1989;37:305-310
24. Takahira N, Itoman M, Higashi K, Uchiyama K, Miyabe M, Naruse K. Treatment outcome of two-stage revision total hip arthroplasty for infected hip arthroplasty using antibiotic-impregnated cement spacer. J Orthop Sci. 2003;8:26-31
25. Von Eiff C. In-vitro-Aktivität von Quinopristin-Dalfopristin gegenüber Staphylokokken und anderen grampositiven Erregern. Chemother J. 2000;19:24-26
26. Wallrauch C, Elsner E, Milatovic D, Cremer J, Braveny I. Antibiotikaresistenz der Enterokokken in Deutschland. Medizinische Klinik. 1997;92:464-468
27. Wendt C, Rüden H, Edmond M. Vancomycin-resistente Enterokokken. Dt Ärztbl. 1998;95:1284-129
28. Wilcox R.R. A review of exact hypothesis testing procedures (and selection techniques) that control power regardless of the variances. Br J Math Stat Psych. 1984;37:34-38
29. Wilde AH, Ruth JT. Two stage implantation in infected total knee arthroplasty. Clin Orthop. 1988;236:23-35
30. Wentworth SJ, Masri BA, Duncan CP, Southworth CB. Hip Prosthesis of Antibiotic-Loaded Acrylic Cement for the Treatment of Infections Following Total Hip Arthroplasty. J Bone Joint Surg. 2002;84:123-128
Author contact
![]() Correspondence to: Dr. med. Jens Kelm, Diplom-Sportlehrer, Chirurgisch-Orthopädisches Zentrum Illingen, Rathausstr. 2, D-66557 Illingen/Saar, Germany. Tel: 0049 6841 1624520; Fax: 0049 6841 1624516; E-Mail: jk66421com
Correspondence to: Dr. med. Jens Kelm, Diplom-Sportlehrer, Chirurgisch-Orthopädisches Zentrum Illingen, Rathausstr. 2, D-66557 Illingen/Saar, Germany. Tel: 0049 6841 1624520; Fax: 0049 6841 1624516; E-Mail: jk66421com

 Global reach, higher impact
Global reach, higher impact