3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(5):241-246. doi:10.7150/ijms.6.241 This issue Cite
Research Paper
Vacuum-assisted closure in the treatment of early hip joint infections
1. Department of Orthopaedic Surgery, University Hospital, Saarland University, Homburg/Saar, Germany
2. Chirurgisch-Orthopädisches Zentrum Illingen/Saar, Germany
Received 2009-8-1; Accepted 2009-9-2; Published 2009-9-2
Abstract
The aim of the present study was to evaluate the efficacy of the vacuum-assisted closure (V.A.C.) system in the treatment of early hip joint infections. 28 patients (11 m / 17 f; mean age 71 y. [43-84]) with early hip joint infections have been treated by means of the V.A.C.-therapy. At least one surgical revision [1-7] has been unsuccessfully performed for infection treatment prior to V.A.C. - application. Pathogen organisms could have been isolated in 22/28 wounds. During revision, cup inlay and prosthesis head have been exchanged and 1-3 polyvinylalcohol sponges inserted into the wound cavity/ periprosthetically at an initial continuous pressure of 200 mm Hg. Postoperatively, a systemic antibiosis was given according to antibiogram. 48-72 h after surgery an alteration from haemorrhagic to serous fluid was observed in the V.A.C.-canister. Afterwards, the pressure was decreased to 150 mm Hg and remained at this level till sponge removal. After a mean period of 9 [3-16] days the inflammation parameters have been retrogressive and the sponges were removed. An infection eradication could be achieved in 26/28 cases. In the two remaining cases the infected prosthesis had to be explanted and a gentamicin-vancomycin-loaded spacer has been implanted, respectively. At a total mean follow-up of 36 [12-87] months no reinfection or infection persistence was observed. The V.A.C.-system can be a valuable contribution in the treatment of early joint infections when properly used. Indications should be early infections with well-maintained soft-tissues for retention of the negative atmospheric pressure.
Keywords: vacuum-assisted closure, V.A.C.-therapy, early infection, hip joint
Introduction
In spite of numerous prophylactic measures infections after primary total hip arthroplasty (THA) still occur in 0.5 - 1.4 % of the cases [4]. Generally, joint infections are categorised in early (within 6 weeks after surgery) and late infections (> 6 weeks) [15]. Efforts for infection management in early cases with prosthesis preservation include debridement, necrosectomy, pulsatile lavage, insertion of antibiotic-loaded device (PMMA-beads, collagen sponges) or flush-suction drain [15]. Should these methods be unsuccessful, a one-stage procedure is usually carried out [1, 15]. Hereby, the infected prosthesis is removed, debridement and jet lavage of the infected area are performed and a new endoprosthesis is reimplanted at the same time. In most of these cases the infection management consists of systemic antibiosis and antibiotic-loaded bone cement for fixation of the prosthesis. The patient is not endangered by the risks of additional surgeries, and the success rates are reportedly between 80-85 % [15].
However, in case of a surgical revision with prosthesis retention, one of the major problems might be wound healing complications with a persistent draining sinus despite revision. In multiple surgical fields the vacuum-assisted closure (V.A.C.) has been established as an efficient treatment option even for deep wound infections. The most important advantages of this system are an increased localised blood flow, the reduction of tissue bacterial counts, elimination of interstitial edema and the safe fluid flow in a closed system [2]. Furthermore, the structure of the sponges and the altered pressure environment in the wound stimulate the proliferation of granulation tissue [2]. However, although the V.A.C.-therapy corresponds with the concept of infection treatment with implant/prosthesis preservation, it is not often used in orthopaedic surgery.
In this study, we report our experience on the management of bacterial infections and/or draining sinus of the hip joint by means of the V.A.C. - therapy.
Patients-Methods
Between 2000 and 2007, 66 patients with early infections after various hip joint surgeries (Fig. 1) have been treated in our department. In 38 cases, an infection eradication could be achieved after a single revision, consisting of hematoma removal, pulsatile lavage and insertion of gentamicin-loaded collagen sponges. The remaining 28 patients (11 male/ 17 female; mean age 71 y. [43 - 84]) have been treated by means of V.A.C.-therapy (Table 1). In all cases, at least one surgical revision [1 - 7] has been performed for infection treatment prior to V.A.C. - application without success. Pathogen organisms could have been isolated in 22/28 wounds. All data about the patients, primary surgical procedures, revisions and follow- up are shown in Table 1.
Patient's data, surgical procedures and causative organisms.
| Patient | Age / Gender | Primary surgery | Pathogen organism(s) | Time period between prim. surgery and revision [weeks] | Time period of V.A.C. implantation [days] | Revisions prior to V.A.C. implantation | Follow-up [months] |
|---|---|---|---|---|---|---|---|
| 1 | 81/ M | aseptic cup | S. marcescens | 3 | 11 | 2 | 68 |
| loosening | CN staphylococci | ||||||
| gram(-) rods | |||||||
| 2 | 51/ F | Girdlestone | E. faecalis | 10 | 13 | 4 | 45 |
| 3 | 46/ M | spacer | MRSA | 3 | 8 | 7 | 87 |
| implantation | |||||||
| 4 | 54/ F | spacer | MRSA | 4 | 11 | 2 | 82 |
| implantation | |||||||
| 5 | 49/ F | Girdlestone | E. faecalis | 7 | 8 | 3 | 67 |
| 6 | 71/ F | aseptic cup | gram (+) anaerobes | 5 | 11 | 1 | 44 |
| loosening | P. magnus | ||||||
| S. epidermidis | |||||||
| 7 | 84/ M | THA after spacer | S. simulans | 3 | 8 | 1 | 43 |
| implantation | |||||||
| 8 | 82/ F | aseptic cup | S. epidermidis | 1 | 13 | 1 | 42 |
| loosening | |||||||
| 9 | 69/ M | primary THA | S. epidermidis | 3 | 16 | 1 | 35 |
| 10 | 51/ F | aseptic cup | Enterobacteriae | 3 | 12/11/13 | 1 | 36 |
| loosening | S. epidermidis | ||||||
| C. albicans | |||||||
| MRSA | |||||||
| 11 | 83/ F | primary THA | E. coli | 4 | 12 | 2 | 32 |
| 12 | 52/ F | THA after spacer | S. epidermidis | 5 | 11 | 2 | 32 |
| implantation | |||||||
| 13 | 71/ F | THA after spacer | P. vulgaris | 2 | 11 | 1 | 32 |
| implantation | E. cloacae | ||||||
| 14 | 57/ M | primary THA | CN staphylococci | 3 | 12 | 1 | 42 |
| 15 | 73/ M | THA after spacer | no bacterium identified | 2 | 9 | 1 | 42 |
| implantation | |||||||
| 16 | 73/ F | primary THA | S. epidermidis | 5 | 10/3/7 | 1 | 42 |
| K. pneumoniae | |||||||
| 17 | 65/ M | primary THA | no bacterium identified | 4 | 9 | 1 | 42 |
| 18 | 49/ F | primary THA | S. aureus | 4 | 7 | 2 | 35 |
| C. albicans | |||||||
| 19 | 71/ M | primary THA | E. faecalis | 1 | 7/9 | 1 | 30 |
| 20 | 79/ F | primary THA | S. aureus | 7 | 7 | 1 | 30 |
| 21 | 72/ F | aseptic stem | S. epidermidis | 2 | 6 | 1 | 25 |
| loosening | |||||||
| 22 | 70/ F | primary THA | E. faecalis | 2 | 4/9 | 1 | 23 |
| K. pneumoniae | |||||||
| P. mirabilis | |||||||
| 23 | 72/ F | aseptic stem | E. faecalis | 2 | 8 | 1 | 20 |
| loosening | |||||||
| 24 | 72/ M | resection of heterotopic ossification after primary THA | no bacterium identified | 2 | 8 | 1 | 20 |
| 25 | 66/ F | tumour prosthesis | no bacterium identified | 4 | 7/5 | 1 | 17 |
| 26 | 43/ M | primary THA | S. aureus | 4 | 5 | 1 | 15 |
| 27 | 79/ F | spacer | no bacterium identified | 2 | 8 | 1 | 13 |
| implantation | |||||||
| 28 | 67/ M | spacer | no bacterium identified | 2 | 9 | 1 | 12 |
| implantation |
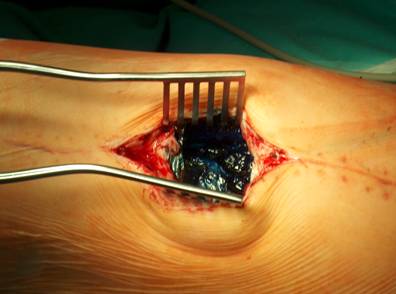
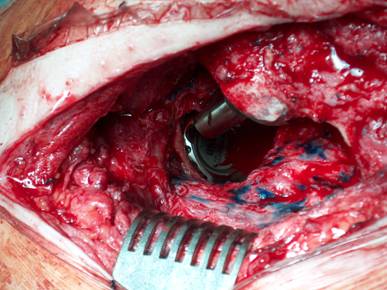
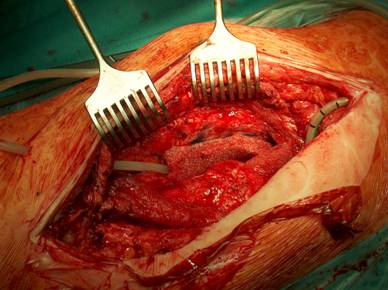
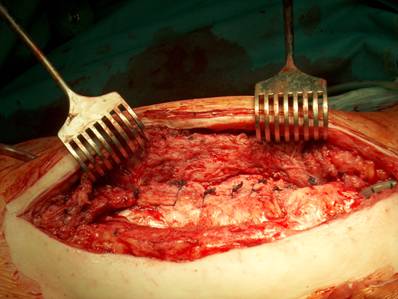
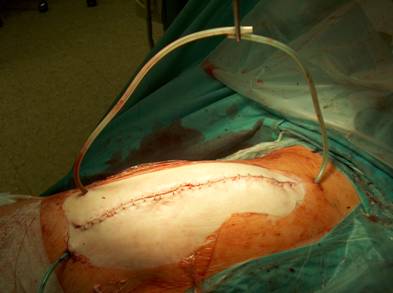
Our operative concept always began by injecting methylene blue into the fistula in order to define the areas of necessary debridement and lavage (Fig. 2). The surgical approach was chosen according to the surgeon's preferences or along the pre-existing surgical approach. All tissue layers were anatomically prepared until reaching the wound/resection cavity or the prosthesis. Meticulous debridement of all necrotic and infected tissues and jet lavage with 10 l Ringer solution PL 2511 (Fresenius-Kabi, Bad-Homburg, Germany) were performed. If possible, cup inlay and prosthesis head were exchanged (Fig. 3). 1-3 polyvinylalcohol (PV) sponges have been placed either around the prosthesis stem or into the resection cavity (Fig. 4) with a transcutan tube outgoing. We chose the PV sponges instead of the polyurethane ones because they cause less pain and can be left in situ for a longer time period. The wounds have been closed in layers under meticulous reconstruction and accurate adaptation of the tissue layers (Fig. 5-6). One redon drain has been placed subcutaneously. Postoperatively, a continuous subatmospheric pressure of 200 mm Hg has been initially attached to the wound via V.A.C.® ATS (KCI, Medizinprodukte GmbH, Walluf, Germany).
After infection eradication (defined by the clinical course, laboratory parameters and inspection of the drained fluid by the V.A.C. - system) the V.A.C. sponges were removed. After pulsatile lavage, 1-2 gentamicin-loaded collagen sponges were inserted into the wound. One redon drain was placed around the prosthesis or into the wound cavity and another was placed subfascial.
Postoperatively, all patients have been treated with systemic antibiosis according to antibiogram for the first 2 weeks followed by an oral antibiosis for another 2 weeks. In cases without germinal proof, a broad spectrum antibiosis (cefuroxime + clindamycin) has been applied.
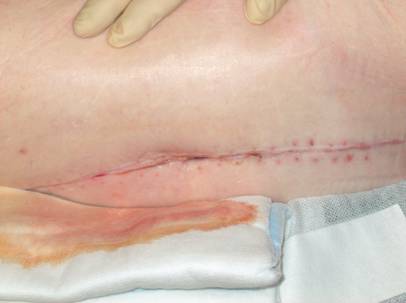
Draining sinus 3 weeks after total hip arthroplasty.
Injection of methylene blue into the fistula for identification of all infected tissues.
Cup inlay and prosthesis head should be removed on routine.
Periprosthetical insertion of polyvinylalcohol sponges.
Anatomical reconstruction and adaptation of the tissue layers for retention of the V.A.C. - therapy.
The wound is closed, the tubes are transcutaneously lead out.
Results
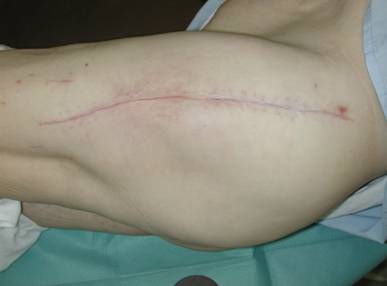
48-72 h after surgery an alteration from haemorrhagic to serous fluid could be observed in the V.A.C. - canister. Afterwards, the continuous pressure was decreased to 150 mm Hg and remained at this level until sponge removal. After a mean period of 9 [3 - 16] days the inflammation parameters (C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), leucocytes blood count) were retrogressive, and the wound secretion was obviously reduced so that we were able to plan the surgical removal of the sponges. At this point, we accurately debrided the skin parts of the tubes exit holes. In cases with a macroscopically not clean operative situs, the sponges were only exchanged and tissue samples as well as parts of the removed V.A.C sponges were sent for further microbiological examination. No complications were observed during the sponges' removal, independent on the implantation period. At sponges removal, tissues samples sent for microbiological examination were negative in 26/28 cases. At a mean follow-up of 36 [12 - 87] months no reinfections occurred (Fig. 7).
Complications were observed in three cases. In case no. 2, it was necessary to remove the sponges after 8 days due to a postoperative haemorrhagia and consecutive coagulation complications caused by liver cirrhosis and consecutive thrombocytopenia. However, there was no negative influence on the remaining therapy progress with regard to the infection sanitation. In cases no. 10 and 16, the infection persisted so that the endoprosthesis had to be removed and a gentamicin-vancomycin-impregnated PMMA spacer (1 g gentamicin/ 4 g vancomycin/ 80 g PMMA) was implanted, respectively. 3 months later, no local or systemic signs of infection could be detected. A prosthesis reimplantation was performed in both cases. At a further follow-up of 30 and 32 months, respectively, no reinfection or infection persistence could be observed.
Outcome 3 months after V.A.C. - therapy.
Discussion
Joint infections are still a hazardous problem in orthopaedic surgery. Infections after total hip replacement are based on the bacterial colonisation on the surface area of an endoprosthesis involving the local tissue and causing an immune reaction [15]. The most common problem hereby is the draining sinus, prolongating and preventing wound healing. The pathophysiological mechanisms are the emergence of interstitial edema, disturbed microcirculation and bacterial contamination, usually leading to prosthesis explantation [15].
Morykwas et al. have demonstrated that the application of the V.A.C. - system increased the granulation tissue formation and the local blood flow, and enhanced the bacterial clearance function [13]. Since interstitial edema is eliminated without any impact on systemic haemodynamics, the surrounding tissue is decompressed and local microcirculation is re-established. The increase of the oxygen gradient facilitates the transportation of toxins and inhibitors inducing thereby the wound healing [16]. As a result of the increased angiogenesis, the local antibiotic concentrations are increased, too [3, 11]. Last but not least, the transport of cellular and humoral components of the immune system to the infected area is facilitated, which leads to a significant reduction of the bacterial count.
The efficacy of the V.A.C. therapy has been proven in the treatment of wound infections. Fleischmann et al. could show that exposed implants can be preserved by V.A.C.-application and an overgrowth of granulation tissue can be observed [5]. Despite its advantages as granulation tissue formation, safe wound fluid flow and bacteria count reduction, the V.A.C.-system is not often used in the treatment of prosthesis - related infections.
Currently, there exist only four reports about the use of the V.A.C. - system in the infection management after total knee or hip arthroplasty. Interestingly, all of them have used only polyvinylalcohol sponges for infection management. Kelm et al. reported an infection eradication in 9 out of 10 patients having early infections after total hip or excision arthroplasty by means of the conventional V.A.C.-system at a mean follow-up of 21 months [6]. Kirr et al. showed good results in 3 cases by using the V.A.C.-Instill® system in the treatment of early infections after total hip replacement (follow-up not reported) [7]. Lehner and Bernd made similar observations with the V.A.C.-Instill® system in 2 cases at a follow-up of 8 and 22 weeks, respectively [10]. Lüdemann et al. treated 17 cases of early infections after THA by insertion of polyvinylalcohol sponges [12]. An infection persistence or reinfection was seen in 8 cases (47 %; follow-up not reported), however, the authors defined early infections as those within the first postoperative year and not within the first six postoperative weeks.
To our knowledge, our patient collective is the largest one having been treated by means of the V.A.C.-therapy at the site of an early hip joint infection. We believe that the careful patients' selection as well as the modified technique is responsible for the good outcome. In the usual V.A.C. - technique, the sponges are fixed on wound areas by a sterile self adhesive foil. In contrast to that, we insert the sponges either periprosthetically or into the resection cavity. Hereby, intact soft-tissues are an indispensable premise for emergence and retention of the vacuum. The tubes supporting the subatmospheric pressure applied are transcutaneously led out. Then the wound is closed. In order to achieve a bacterial count reduction, the following requirements have to be met: meticulous debridement of the tissue [16-17], mechanical cleaning and lavage of the exposed prosthesis parts. Moreover, we advance the view that an adequate wound closure can only be performed after an exact anatomical preparation and mobilisation of the tissue layers. This anatomical preparation and the resulted reconstruction of the soft-tissue layers may allow an enhanced biomechanical function for the postoperative clinical outcome. Hereby, the tissue blood flow is optimised, thus contributing to infection sanitation [11]. Further advantages of our procedure are the less extensive bandage changing, with a positive influence on the patient´s convenience [16] and the possibility of costs minimization [8, 14]. The disadvantage of lacking inspections of the sponges can be compensated by daily controlling of the canister fluid and monitoring of the inflammation parameters.
Our clinical outcome with a success rate in 26 out of 28 cases after a mean follow-up of 3 years indicates that the V.A.C.-therapy can be a valuable contribution to the treatment of early hip joint infections. However, indications for V.A.C. application should be strictly made. If a vacuum cannot be developed due to skin and tissue lesions or necroses, then the V.A.C.-therapy should not be used for infection management of the underlying implant. Moreover, the virulence of the causative pathogens, the severity of infection and the host response to antimicrobial treatment are parameters to bear in mind when V.A.C.-therapy is elected for infection management. Multimicrobial infections, including bacteria and fungi [9], seem to overstrain the clearance function of the V.A.C.-therapy, thus being probably the reason for the system failure in the infection treatment in case 10.
One limitation of our study is that we cannot define the ideal frequency for sponge exchange since there exists no consensus regarding the use of the V.A.C. - therapy in orthopaedic surgery. Most concepts are based on subjective experiences gained over the years. Moreover, it is unknown whether the polyurethane sponges of the V.A.C. - system would have the same outcome as the polyvinylalcohol sponges did in the present study. Equally to that, our results account only for an initial pressure of -200 mm Hg with a reduction after 2-3 days to -150 mm Hg. It is unknown if other pressure heights would have a similar outcome. Moreover, our study does not have a control group treated by another regime. However, patients' collectives suffering from joint infections are frequently so inhomogenous that a randomization is difficult, and therefore, the orthopaedic surgeon should treat each case individually.
In conclusion, we recommend that the V.A.C.-therapy can be used in cases with prolonged draining sinus or early infections after total hip and excision arthroplasty. Intact soft-tissues are an indispensable premise for development and retention of the vacuum. The patients' comfort can be enhanced and the frequency of the bandages exchange reduced. However, its application in cases with high virulent organisms is arguable. Hereby, the creation of a therapy concept is essential with respect to the treatment strategies of septic surgery and the causes of the infection.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Alfaro-Adrian J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14:439-45
2. Banwell PE. Topical negative pressure therapy in wound care. J Wound Care. 1999;8:79-84
3. Bourée M, Kozianka J. Die V.A.C. Therapie in der Allgemeinchirugie. European Society ACA. 2003;191(Suppl 35):35-38
4. Cramer J, Ekkernkamp A, Ostermann PA. The infected endoprosthesis with the example of the hip joint endoprosthesis. An increasing danger to patient and society. Z Arztl Fortbild Qualitatssich. 2001;95:195-201
5. Fleischmann W, Strecker W, Bombelli M, Kinzl L. Vacuum sealing as treatment of soft tissue damage in open fractures. Unfallchirurg. 1993;96:488-92
6. Kelm J, Anagnostakos K, Schmitt E. Closed subfascial V.A.C.-therapy in periprosthetic hip infections. Zentralbl Chir. 2004;129:S49-52
7. Kirr R, Wiberg J, Hertlein H. Clinical experience and results of using the V.A.C. instill therapy in infected hip- and knee prosthetics. Zentralbl Chir. 2006;131:S79-82
8. Langley-Hawthorne C. Economics of negative pressure wound therapy. Ostomy Wound Manage. 2004;50(4A Suppl):35-7
9. Lazzarini L, Manfrin V, De Lalla F. Candidal prosthetic hip infection in a patient with previous candidal septic arthritis. J Arthroplasty. 2004;19:248-52
10. Lehner B, Bernd L. V.A.C.-Instill therapy in periprosthetic infection of hip and knee arthroplasty. Zentralbl Chir. 2006;131:S160-4
11. Loree S, Dompmartin A, Penven K, Harel D, Leroy D. Is Vacuum Assisted Closure a valid technique for debriding chronic leg ulcers? J Wound Care. 2004;13:249-52
12. Lüdemann M, Haid S, Wülker N, Rudert M. Results of vacuum sealing in joint infections. Z Orthop Ihre Grenzgeb. 2006;144:602-8
13. Morykwas MJ, Argenta LS, Shelton-Brown EI, McGuirt W. Vacuum-Assisted-Closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553-62
14. Neubauer G, Ujlaky R. The cost-effectiveness of topical negative pressure versus other wound-healing therapies. J Wound Care. 2003;12:392-3
15. Steinbrink K, Frommelt L. Treatment of periprosthetic infection of the hip using one-stage exchange surgery. Orthopäde. 1995;24:335-43
16. Webb LX, Schmidt U. Wound management with vacuum therapy. Unfallchirurg. 2001;104:918-926
17. Webb LX. New techniques in wound management: Vacuum-Assisted Wound Closure. J Am Acad Orthop Surg. 2002;10:303-311
Author contact
![]() Correspondence to: Dr. Konstantinos Anagnostakos, Klinik für Orthopädie und Orthopädische Chirurgie, Universitätskliniken des Saarlandes, Kirrbergerstr. 1, D-66421, Homburg/Saar, Germany. Tel.: 0049-6841-1624520; Fax: 0049-6841-1624516; e-mail: k.anagnostakosde
Correspondence to: Dr. Konstantinos Anagnostakos, Klinik für Orthopädie und Orthopädische Chirurgie, Universitätskliniken des Saarlandes, Kirrbergerstr. 1, D-66421, Homburg/Saar, Germany. Tel.: 0049-6841-1624520; Fax: 0049-6841-1624516; e-mail: k.anagnostakosde

 Global reach, higher impact
Global reach, higher impact