3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2009; 6(1):37-42. doi:10.7150/ijms.6.37 This issue Cite
Research Paper
Continuous Non-Invasive Arterial Pressure Technique Improves Patient Monitoring during Interventional Endoscopy
Department of Internal Medicine I, University of Regensburg, Germany
Received 2008-11-17; Accepted 2009-1-10; Published 2009-1-20
Abstract
Introduction: Close monitoring of arterial blood pressure (BP) is a central part of cardiovascular surveillance of patients at risk for hypotension. Therefore, patients undergoing diagnostic and therapeutic procedures with the use of sedating agents are monitored by discontinuous non-invasive BP measurement (NIBP). Continuous non-invasive BP monitoring based on vascular unloading technique (CNAP®, CN Systems, Graz) may improve patient safety in those settings. We investigated if this new technique improved monitoring of patients undergoing interventional endoscopy.
Methods: 40 patients undergoing interventional endoscopy between April and December 2007 were prospectively studied with CNAP® in addition to standard monitoring (NIBP, ECG and oxygen saturation). All monitoring values were extracted from the surveillance network at one-second intervals, and clinical parameters were documented. The variance of CNAP® values were calculated for every interval between two NIBP measurements.
Results: 2660 minutes of monitoring were recorded (mean 60.1±34.4 min/patient). All patients were analgosedated with midazolam and pethidine, and 24/40 had propofol infusion (mean 90.9±70.3 mg). The mean arterial pressure for CNAP® was 102.4±21.2 mmHg and 106.8±24.8 mmHg for NIBP. Based on the first NIBP value in an interval between two NIBP measurements, BP values determined by CNAP® showed a maximum increase of 30.8±21.7% and a maximum decrease of 22.4±28.3% (mean of all intervals).
Discussion: Conventional intermittent blood pressure monitoring of patients receiving sedating agents failed to detect fast changes in BP. The new technique CNAP® improved the detection of rapid BP changes, and may contribute to a better patient safety for those undergoing interventional procedures.
Keywords: continuous non-invasive blood pressure, procedural sedation, endoscopy, cardiovascular monitoring, hypotension.
Introduction
Cardiovascular complications including arrhythmia, ischemia and hypotension during interventional endoscopy, are not common, but nevertheless higher than previously reported, and may cause harm to patients [1, 2]. In elderly patients and in those with compromised cardiovascular function even short episodes of hypotension may cause extensive problems. Hence close monitoring of the arterial blood pressure (BP) is a central part of cardiovascular surveillance in these patients. Theoretically, this is guaranteed at best by invasive monitoring with an intra-arterial catheter, but this would put patients at risk for adverse events like infections or necrosis. Therefore, patients undergoing diagnostic and therapeutic procedures with the use of sedating agents are monitored by discontinuous non-invasive BP measurement (NIBP) with measure intervals between 3 to 15 minutes as standard. However, hypotensive episodes may be missed between these intervals. One possible solution may be the application of a new technique of continuous non-invasive BP monitoring by CNAP® (CN Systems, Graz, Austria) in those settings. CNAP® is based on the vascular unloading technique and enables a beat-to-beat BP measurement without having substantial negative side effects [3, 4]. We compared test readings from NIBP and CNAP® values in patients undergoing interventional endoscopy such as endoscopic retrograde cholangiopancreatography (ERCP). The aim of this study was to investigate the accuracy of NIBP measurements in these patients, and if cardiovascular patient monitoring in interventional endoscopy could be improved by CNAP®.
Methods
Study design
The prospective study took place between April and December 2007 on patients undergoing interventional endoscopy. The study was approved by the ethics committee of the University of Regensburg and performed in accordance with the declaration of Helsinki.
Patients
40 patients undergoing interventional endoscopy were monitored by CNAP® in addition to standard monitoring (NIBP, ECG and oxygen saturation). Patients with peripheral vascular pathology like vascular implants and raynaud syndrome were excluded. All patients were treated with analgosedative medication and were asked to limit their arm movements.
Monitoring values were extracted from the surveillance network at one-second intervals by using e-data® software (Draeger medical solutions, Lübeck, Germany). Additionally clinical and demographical parameters were recorded.
Materials
Continuous non-invasive BP monitoring based on vascular unloading technique (CNAP®, CN Systems, Graz, Austria) is commercially available since 2007. It can be used in combination with Task Force Monitor® (CN Systems, Graz, Austria) or with Draeger (Draeger Medical, Lübeck, Germany) and Siemens Monitors (Siemens Erlangen, Germany). The method is based on concentrical interlocking control loops for correct long-term tracing of finger BP, including automatic set point adaption, light control and separate inlet and outlet valves for electric-pneumatic control [3, 4]. The cuff pressure is continuously changed through the systolic and diastolic blood pressure cycle to keep the finger's luminescence constant. Therefore, the cuff pressure corresponds to the pressure in the finger at any time. CNAP® is calibrated by standard NIBP via upper arm's cuff.
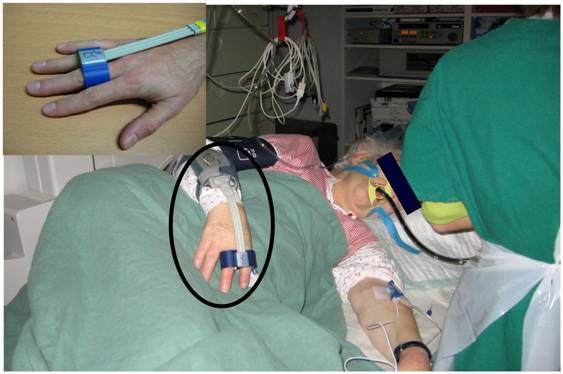
Figure 1 shows the double-finger cuff, placed at patient's middle and index finger or middle and ring finger; respectively. The cuff is connected with the cuff controller and the monitoring device.
Interventional endoscopy in our gastroenterological department; in the left corner the CNAP® double-finger module is highlighted.
Statistic analysis
Data collection and statistical calculations were performed using Microsoft Office Access (Version 2007, Microsoft Corporation, Redmond, USA) and SPSS® (SPSS inc, Version 15, Germany). Data are expressed as mean + SD.
Results
Patients
24 female and 16 male patients, with an average age of 59±15 years underwent 32 ERCPs and 8 other interventional endoscopies with a mean duration of 66 minutes ± 34 minutes. Indications leading to endoscopy were: malignant stenosis of the hepatic duct (n=13), cholelithiasis (n=8), benign stenosis of the hepatic duct (n=6), pancreatitis/pancreas-pseudocysts (n=4), others (n=8). All patients (mean BMI 25 kg/m²) received midazolam (7.4±3.3 mg) and pethidin (52.5±19.2 mg), 24 patients were additionally treated with propofol (90.9±70.3 mg) and 2 patients with ketamine (100.0±50.0 mg). No cardiovascular complications following endoscopy were detected.
NIBP and CNAP® values
2660 minutes of monitoring were recorded. Within this time 103 117 CNAP® and 333 NIBP measurements were recorded. Furthermore, we recognized 143 088 heart rate values and 145 665 oxygen saturation values.
The mean NIBP arterial pressure was 106.82±24.82 mmHg (mean arterial pressure). On average, 10.3 NIBP measurements were performed per endoscopy. The mean arterial pressure values determined by CNAP® were 102.37±21.20 mmHg and therefore not significantly different from the mean NIBP values.
In order to determine blood pressure changes undetected by conventional NIBP surveillance, NIBP intervals were defined as the time space between two adjacent NIBP measurements, and the CNAP readings within these intervals were analyzed (figure 2). In total, 254 NIBP intervals were calculated with a mean length of 7.5±4.6 minutes.
The variance of CNAP® values for every NIBP interval based on the first NIBP value
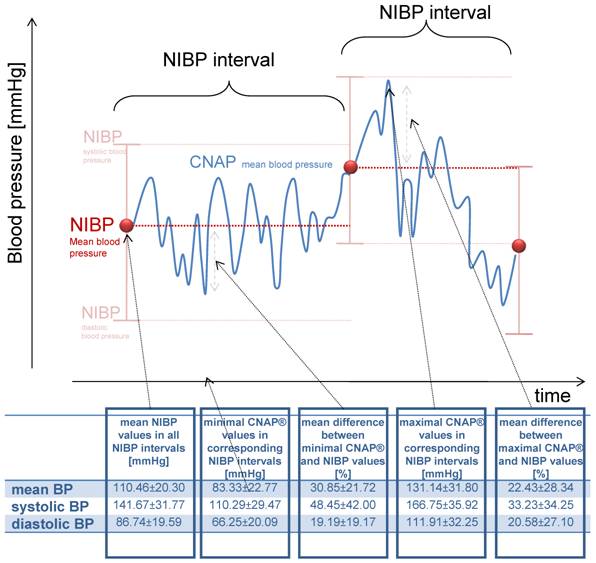
Using standard cardiovascular monitoring, a NIBP value is presumed to reflect the patient's blood pressure until a second value is available. Therefore, we calculated the variance of CNAP® values for every NIBP interval based on the first NIBP value (figure 2). With this approach, the maximal increase and decrease in blood pressure was calculated for each interval, and the mean of these deviations for all NIBP intervals was determined. The mean maximum decrease was 27.13±16.81 mmHg (30.85%) and the mean maximal increase was 20.69±28.34 mmHg (22.43%) per NIPB interval (figure 2).
Most physicians using NIBP monitoring during procedural sedation are conscious of the fact that arterial pressure values may differ between two NIBP measurements. We assumed that a fluctuation of 10% or 20% of the initial NIBP value can be safely tolerated, depending on the initial value (figure 3). In our investigation, 45.12% of all mean CNAP® values were beyond this “tolerable” interval of 10%, and 15.80% of the values were even beyond the 20% range.
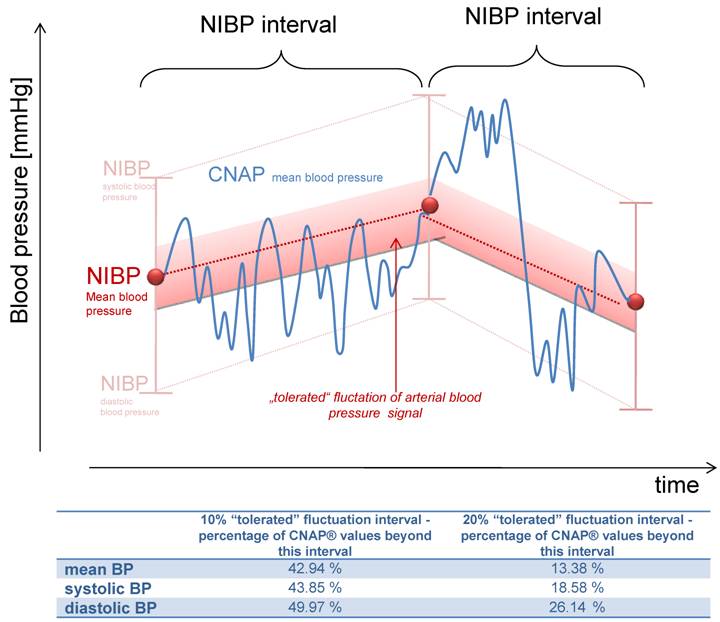
The described deviations of the CNAP blood pressure from the first NIBP value might result from a continuous rise or fall to the next measured NIBP value (figure 4). To evaluate if this accounts for the deviations described or if there is more fluctuation in blood pressure, straight lines between two NIBP values were calculated and tolerance intervals of ± 10% and 20% were set (figure 4). 42.94% of all CNAP® values (mean BP) were outside the 10% interval and 13.38% of the values outside the 20% corridor, demonstrating a profound fluctuation of blood pressure values which were not detected by NIBP values. In clinical practice, detection of hypotensive episodes is more important than the fluctuation of blood pressure values. In our data base, none of the systolic blood pressure values were lower than 100 mmHg, but with CNAP, 3.6% of the registered values were below 100 mmHg. Therefore, continuous blood pressure surveillance has the potential to detect hypotension earlier than NIBP measurements, and may improve patient safety.
Illustration of the "tolerated" fluctuation of 10% or 20% respectively, based on the initial NIBP value. The percentage of measurements within this corridor is shown bellow.
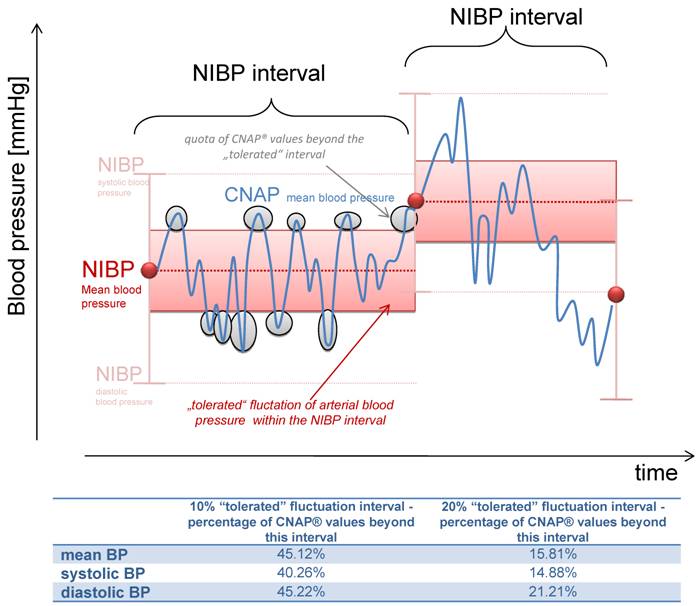
Illustration of the "tolerated" fluctuation of 10% or 20% respectively, based on the calculated straight lines between two NIBP values. The percentage of measurements within this corridor is shown bellow.
Discussion
Patients undergoing interventional endoscopy, as a common example of procedural analgo-sedation, require close cardiovascular monitoring due to well known complications of sedative agents like hypotension and respiratory depression [5]. The use of propofol for procedural sedation is increasing because of its rapid onset and offset properties [5], although an increased rate of fatal complications was published [6] and its use in endoscopy is controversially discussed by gastroenterologists and anesthesiologists [7, 8]. Especially the rate of hypotension measured by the method of Riva-Rocci has been reported as high as 8-12%, although many publications showed the safety of propofol for procedural sedation in endoscopy [9, 10] and in emergency departments [11, 12].
Our results using a continuous non-invasive BP monitoring by vascular unloading technique (CNAP®) during interventional endoscopy show that there are large BP changes in between the currently common discontinuous NIBP measurements, and 16% of mean CNAP® values differed more than 20% from the previous NIBP value. In our study only a few episodes of hypotension were detectable, but none of these were registered by NIBP measurements. In other collectives, the rate of hypotensive episodes may be higher. Our data show that the rate of hypotension previously determined by NIBP measurements underestimates the true hypotension incidence during endoscopy, and especially rapid BP chances are often undetected. Unfortunately, the local ethics committee did not permit blinding of the CNAP values to the endoscopists, which may explain the quite large NIBP intervals, thereby underestimating the accuracy of the NIPB measurements.
The clinical impact of hypotensive episodes during procedural sedation is not entirely clear, since short hypotensive episodes in general anesthesia are rarely been associated with permanent problems [13]. However, rapid BP changes may be responsible for considerable side effects. Cardio-pulmonary complications account for the majority of all reported complications during endoscopy [14], [1]. Besides well-known serious conditions like respiratory failure and cardiac arrest, hypotensive episodes in the cardiovascular compromised patient may result in ischemic complications such as kidney failure or heart ischemia [1, 2, 15]. In our study, no serious complications occurred, but due to the study design the medical staff was aware of the measured CNAP® blood pressure values throughout the study.
Our study provides evidence that hypo- and hypertensive episodes are earlier recognized with a continuous noninvasive blood pressure monitoring device, and usage of CNAP® may therefore enable medical staff to act more rapidly and effectively to BP changes before serious adverse events occur. In our opinion, this will help to perform procedural sedation more safely.
Conclusion
Cardiovascular monitoring by CNAP® detects rapid changes in blood pressure occurring surprisingly often during procedural sedation and analgesia in interventional endoscopy, and may therefore improve patient safety.
Conflict of Interest
The CNAP® equipment was provided by CN Systems (Graz, Austria). No other conflict of interest exists.
References
1. Arrowsmith JB, Gerstman BB, Fleischer DE, Benjamin SB. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421-427
2. Gangi S, Saidi F, Patel K, Johnstone B, Jaeger J, Shine D. Cardiovascular complications after GI endoscopy: occurrence and risks in a large hospital system. Gastrointest Endosc. 2004;60(5):679-685
3. Fortin J, Habenbacher W, Heller A, Hacker A, Grullenberger R, Innerhofer J, Passath H, Wagner C, Haitchi G, Flotzinger D. et al. Non-invasive beat-to-beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Comput Biol Med. 2006;36(11):1185-1203
4. Fortin J, Marte W, Grullenberger R, Hacker A, Habenbacher W, Heller A, Wagner C, Wach P, Skrabal F. Continuous non-invasive blood pressure monitoring using concentrically interlocking control loops. Comput Biol Med. 2006;36(9):941-957
5. Ross C, Frishman WH, Peterson SJ, Lebovics E. Cardiovascular considerations in patients undergoing gastrointestinal endoscopy. Cardiol Rev. 2008;16(2):76-81
6. Tramer MR, Moore RA, McQuay HJ. Propofol and bradycardia: causation, frequency and severity. Br J Anaesth. 1997;78(6):642-651
7. Koch ME, Gevirtz C. Propofol may be safely administered by trained nonanesthesiologists. Con: Propofol: far from harmless. Am J Gastroenterol. 2004;99(7):1208-1211
8. Vargo JJ. Propofol may be safely administered by trained nonanesthesiologists. Pro: Propofol demystified: it is time to change the sedation paradigm. Am J Gastroenterol. 2004;99(7):1207-1208
9. Wehrmann T, Kokabpick S, Lembcke B, Caspary WF, Seifert H. Efficacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc. 1999;49(6):677-683
10. Cohen LB, Dubovsky AN, Aisenberg J, Miller KM. Propofol for endoscopic sedation: A protocol for safe and effective administration by the gastroenterologist. Gastrointest Endosc. 2003;58(5):725-732
11. Zed PJ, Abu-Laban RB, Chan WW, Harrison DW. Efficacy, safety and patient satisfaction of propofol for procedural sedation and analgesia in the emergency department: a prospective study. Cjem. 2007;9(6):421-427
12. Frank LR, Strote J, Hauff SR, Bigelow SK, Fay K. Propofol by infusion protocol for ED procedural sedation. Am J Emerg Med. 2006;24(5):599-602
13. Block FEJr. Normal fluctuation of physiologic cardiovascular variables during anesthesia and the phenomenon of "smoothing". J Clin Monit. 1991;7(2):141-145
14. Osinaike BB, Akere A, Olajumoke TO, Oyebamiji EO. Cardiorespiratory changes during upper gastrointestinal endoscopy. Afr Health Sci. 2007;7(2):115-119
15. Murray AW, Morran CG, Kenny GN, Macfarlane P, Anderson JR. Examination of cardiorespiratory changes during upper gastrointestinal endoscopy. Comparison of monitoring of arterial oxygen saturation, arterial pressure and the electrocardiogram. Anaesthesia. 1991;46(3):181-184
Author contact
![]() Correspondence to: Sylvia Siebig, MD, Department of Internal Medicine I, University of Regensburg, D-93042 Regensburg, Germany, Tel.: +49-941/944-7010; Fax: +49-941/944-7021; E-Mail: Sylvia.siebiguni-r.de
Correspondence to: Sylvia Siebig, MD, Department of Internal Medicine I, University of Regensburg, D-93042 Regensburg, Germany, Tel.: +49-941/944-7010; Fax: +49-941/944-7021; E-Mail: Sylvia.siebiguni-r.de

 Global reach, higher impact
Global reach, higher impact