3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2008; 5(6):361-365. doi:10.7150/ijms.5.361 This issue Cite
Research Paper
Allele dependent silencing of COL1A2 using small interfering RNAs
Dept. of Medical Sciences, Uppsala University, Uppsala, Sweden.
Received 2008-9-29; Accepted 2008-11-10; Published 2008-11-12
Abstract
Osteogenesis imperfecta (OI) is generally caused by a dominant mutation in Collagen I, encoded by the genes COL1A1 and COL1A2. To date there is no satisfactory therapy for OI, but inactivation of the mutant allele through small interfering RNAs (siRNA) is a promising approach, as siRNAs targeting each allele of a polymorphism could be used for allele-specific silencing irrespective of the location of the actual mutations. In this study we examined the allele dependent effects of several tiled siRNAs targeting a region surrounding an exonic COL1A2 T/C polymorphism (rs1800222) in heterozygous primary human bone cells. Relative abundances of COL1A2 alleles were determined by cDNA sequencing and overall COL1A2 abundance was analyzed by quantitative PCR. One of the siRNAs decreased overall COL1A2 abundance by 71% of which 75% was due to silencing of the targeted T-allele. In conclusion, allele-preferential silencing of Collagen type I genes may be a future therapeutic approach for OI.
Keywords: COL1A2, allele-preferential silencing, Osteogenesis imperfecta
INTRODUCTION
Osteogenesis imperfecta (OI) is a heterogeneous disease of the connective tissue with an incidence of approximately 1/10 000. The principal sign of OI is fragile bones with multiple fractures, but the disease can affect many other tissues as well. The mildest form of OI (type I) is often due to a null allele mutation (1), while severe and lethal forms (type II-VII) generally have a qualitative collagen defect (2). More than 90% of OI is caused by a dominant mutation in collagen type I, which is the most abundant protein in connective tissue. Approximately 90% of the organic matrix of bone consists of collagen I, where it provides both the framework for mineralization and the tensile strength that gives bone elasticity. Collagen I is comprised of two α1(I) chains and one α2(I) chain, encoded by the genes COL1A1 and COL1A2, respectively. The three monomers twist together in a zipper like fashion to create a triple helix which has a highly repetitive structure, (Gly-X-Y)n, with the glycine residue at every third position facing the confined space in the centre of the helix. The most common cause of OI is a mutation affecting a glycine residue.
To date, there is no satisfactory therapy for patients with OI. Many patients are treated with bisphosphonates, which there is some support for in some clinical trials (3). However, the results are insufficient and little is known about which patients benefit from this treatment and which do not. It is not known if treatment with other osteoporosis drugs would be a better alternative or would potentially complement the bisphosponate treatment in patients with OI. Considering mutations in severe OI act in a dominant fashion, a therapeutic vision is to convert a severe OI type to a type I OI by silencing the mutated allele. For COL1A1, this would convert a severe phenotype to a mild OI type I, while individuals who are heterozygous for null mutations in COL1A2 are phenotypically normal (4). One attractive avenue is allele specific silencing through RNA interference (RNAi), which in contrast to other methods of manipulation has a high and specific inhibition (5).
RNA interference is the process by which double stranded exogenous RNA elicit degradation of cellular RNA with sequence complementary to one of the strands. Following findings that antisense RNA decreased abundance of complementary mRNA (6) and later discoveries by Fire et al. (7), RNA interference has been developed into an extensively used method to decrease the abundance of specific genes. In 1999, small interfering RNAs (siRNAs) were discovered as endogenous molecules mediating RNA interference in plants (8) and in 2001 it was shown that exogenous double stranded siRNAs efficiently reduced mRNA levels in animal cells in vitro (9). Since these seminal discoveries, the siRNA technology has been further developed and siRNAs are now invaluable as they enable partial gene knockout in vitro as well as in vivo (10).
Recent studies have reported successful allele specific gene silencing by siRNAs able to discriminate between single nucleotide variants within mRNAs (11-13). These studies suggest that siRNAs may be interesting to explore as therapeutics in monogenic dominant disorders such as OI, where the dysfunctional allele could be targeted specifically. Indeed, allele-preferential suppression mediated by RNAi has been described in vitro for human COL1A1 allele constructs transfected into the primate kidney cell line COS-7 and for endogenous COL1A1 in human mesenchymal progenitor cells (14). Additionally, a splice-site mutated COL1A2 allele has been preferentially silenced in fibroblasts from a patient suffering from a type IV OI (15).
To date over 800 mutations have been described as causative of OI (2), making it labour intensive to design siRNAs for every separate mutation. In heterozygous individuals for a common polymorphism, siRNAs targeting each allele of COL1A2 as well as COL1A1 could be used for allele specific silencing irrespective of the location of the actual mutations. In this study we have examined the allele dependent effects of seven tiled siRNAs targeting a region surrounding an exonic COL1A2 SNP (rs1800222), for which the cells were heterozygous.
MATERIALS AND METHODS
siRNA design
Seven tiled 21 nucleotide long siRNAs were designed. Each siRNA had antisense strands (AS) perfectly complementary to the T-allele of rs1800222 (Figure 1). siRNAs were purchased from Ambion as double stranded RNA molecules. Each strand of siRNAs had a two-basepair overhang in the 3'-end (always UU for sense strand) (Figure 1 illustrates the active antisense strand). Negative control siRNAs were purchased from Invitrogen and were: Stealth™ RNAi Negative controls (part numbers: NC1: 12935-200, NC2: 12935-112 and NC3: 12935-110).
Seven tiled siRNAs designed to target the region surrounding the T/C single nucleotide polymorphism (SNP) rs1800222 in the COL1A2 gene. Capital letters visualize 19 nucleotides of the antisense siRNA strand that are perfectly complementary to the T-allele of rs1800222. Each siRNA-strand had a two-nucleotide 3-prime overhang, which is visualized as non-capital letters in the antisense strands of siRNAs 1-7.

Cell culture and transfection
Primary cultures of bone derived cells from patients undergoing hip- and knee replacement surgery were genotyped for a C/T single nucleotide polymorphism (SNP) in exon 6 of COL1A2 (SNP ID rs1800222) (Allele frequencies: T=0.09 A=0.91). Cells from a heterozygous individual were transfected in 24-well cell plates using Magnet Assisted Transfection (MATRA) (Promokine, Germany). In the initial experiment 75,000 cells were seeded the day prior to transfection which was carried out using either 0.6µg of each siRNA, negative control siRNA, vehicle (only magnetic beads) or untreated control cells, with each treatment performed in duplicate wells. Transfected cells were incubated at 37°C in 5% CO2 until RNA was isolated at 48 hours post- transfection. In the subsequent experiment, 17,000 cells were seeded three days prior to transfection using 0.3µg, 0.45µg and 0.6µg of siRNA3 or negative control siRNAs (four wells per treatment). The cells were then incubated for 72 hours until RNA was prepared.
RNA preparation and cDNA-synthesis
RNA was prepared using the QiaShredder kit and the RNeasy mini kit (Qiagen, Germany). Each individual RNA-sample was subjected to DNase treatment using TURBO-DNAfree (Ambion) and equal amounts of RNA were then reversely transcribed with the High Capacity cDNA reverse transcription kit (Applied Biosystems).
Polymerase Chain Reaction and sequencing
Polymerase Chain Reaction (PCR) was used to amplify exons 6 and 25 of COL1A2. The primers used were: Exon 6 forward primer: 5'CCTACCAACATGCCAATCTTTAC, Exon 6 reverse primer: 5'GTTTTCCAGGGTGACCATCTT, Exon 25 forward primer: 5'-AGTCCGAGGACCTAATGGAGAT, Exon 25 reverse primer: 5'-GCATGACCTTTATCACCGTTTT. PCR reactions were performed using standard PCR conditions with an annealing temperature of 60°C. Sequencing PCR reactions were performed using the same primers with BigDye 3.1 sequencing chemistry according to the manufacturers instructions (Applied Biosystems).
Assessment of relative allele abundance of COL1A2 mRNA
The software PeakPicker (17) was used to quantify ratios of the two COL1A2 alleles for all cDNA-samples Briefly, for each individual cDNA-sequence, SNP peak-heights were normalized for peak heights of adjacent non-polymorphic positions. For all treatments, allele ratios of the two SNPs rs1800222 and rs412777 were compared to peak-heights of negative control siRNAs.
Quantitative PCR
Quantitative PCR (qPCR) reactions were performed using ten µl 2x TaqMan® Universal PCR Master Mix, No AmpErase® UNG (Applied Biosystems) was mixed with 9 µl diluted cDNA and 1 µl of Taq-man gene specific assay mix COL1A2: Hs01028967_g1, GAPDH: Hs99999905_m1 (Applied Biosystems). This mix was subjected to 40 cycles of PCR using the ABI Prism 7900 Taqman instrument (Applied Biosystems). Each individual sample was analyzed in duplicate and COL1A2 abundance was normalized relative to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels.
RESULTS
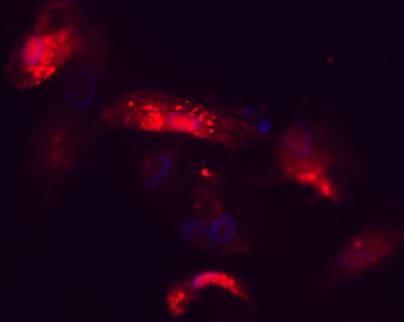
To verify the successful delivery of small RNA, Cy3-labeled negative control siRNAs were transfected to primary bone cells at the same concentration as were used in the silencing experiments. Successful delivery to the target cells is shown in Figure 2, which depicts a fluorescence microscopy image capture of the Cy3-siRNA transfected cells 72 hours post-transfection.
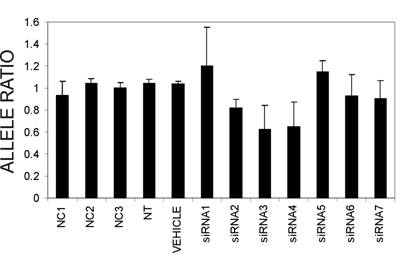
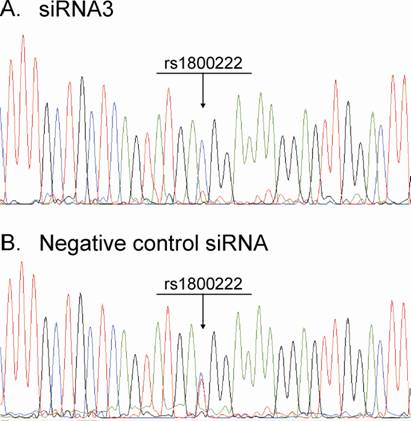
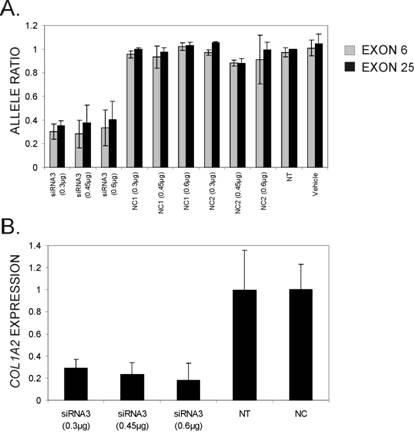
From the silencing experiments using seven tiled siRNAs it was observed that siRNAs 3 and 4 were induced the highest degree of allele-preferential COL1A2 degradation (Figure 3). In a subsequent experiment cells were transfected with three different concentrations of siRNA3, which resulted in a substantial reduction in rs1800222 T/C allele ratio of mRNA in some of the transfected wells (Figure 4). The 0.3µg dose rendered a mean rs1800222 T/ C allele ratio of 33%, and the corresponding ratios for 0.45µg and 0.6µg were 0.30 and 0.35, respectively (Figure 5 A). These results were verified by cDNA sequence analysis of a heterozygous SNP in exon 25 (rs412777) where the allele ratios were 0.35, 0.38 and 0.41 for 0.3µg, 0.45µg and 0.6µg dosages of siRNA3, respectively (Figure 5A). Quantitative PCR analysis revealed that with increasing siRNA3 dosage, COL1A2 abundance was decreased by 71%, 77% and 82% (Figure 5B), of which 75%, 75% and 73% could be attributed to silencing of the targeted T-allele, respectively.
Fluorescence microscope image of Cy3-labeled negative control siRNAs inside of primary bone cells 72 h post-transfection. Red colour indicates areas where siRNAs are present and blue regions depict cellular nuclei stained with DAPI.
mRNA ratios of the two COL1A2 alleles (allele targeted by siRNAs vs. non-targeted allele) 48 hours post-transfection with seven tiled siRNAs targeting the the T-allele of the COL1A2 exon 6 SNP rs1800222. Shown are mean ratios and standard deviations, derived from PeakPicker analysis of cDNA chromatogram peak heights of two heterozygous SNPs in the COL1A2 gene (rs1800222 and rs412777).
Chromatogram from sequencing of cDNA samples derived from RNA isolated 72h post-transfection with: (A) siRNA3. (B) Negative Control siRNA.
Allele ratios of the two COL1A2 mRNA alleles (normalized cDNA peak heights of targeted vs. non-targeted allele of rs1800222) 72 hours post-transfection with three different concentrations of siRNA3. Colours of bars indicate the SNP used to calculate allele ratios from cDNA chromatograms in the software PeakPicker and error bars indicate standard deviations. (B) Relative overall COL1A2 mRNA levels following siRNA treatment quantified by real-time PCR. Expression levels were normalized for GAPDH levels and are presented relative to COL1A2 mRNA levels in cells treated with the negative control siRNAs (NC1 and NC2). Error bars indicate standard deviations.
DISCUSSION
OI is a severe genetic disease with no existing effective or curative treatment. This study was aimed at exploring a genetic therapeutic approach for treating or limiting the severity of this disease. The principle of allele specific silencing of Collagen type I genes has been explored previously by Millington-Ward (14) who reported allele-preferential silencing of COL1A1 in COS7 cells and in primary human mesenchymal progenitor cells. The results reported by Millington-Ward can be regarded as proof of principle for the RNAi approach in OI treatment.
In this study we analyzed both alleles of COL1A2 simultaneously in primary bone cells from a single heterozygous individual and concluded that allele-preferential silencing is possible. Results revealed that the 0.3µg dose of siRNA3 was as specific for the T-allele as the 1.5x and 2x higher concentrations, while seemingly exhibiting less spill-over silencing of the C-allele, signifying that concentration is pivotal for allele specificity. Although the transfection efficiency was not determined we show that fluorescently labelled negative control siRNAs were delivered to the bone cells when cells were transfected with the highest siRNA concentration that was used in the silencing experiments. In future studies it will be necessary to determine the appropriate vehicle for efficient and specific delivery of the allele-preferential siRNAs to the intended target cells in vitro, and ultimately in vivo.
Several hurdles remain to be overcome before truly allele specific siRNAs, which render 50% overall silencing of the Collagen 1 alpha genes, can be tested in clinical trials. The efficiency and specificity of RNA interference using siRNAs is heavily dependent on the base composition of target sites in mRNA as well as on the siRNA sequences themselves. It will be necessary to analyze allele specificity and off-target effects of a large siRNA subset which target the full array of COL1A1 and COL1A2 polymorphisms for which the minor allele occurs in high enough frequencies. In addition to reducing target gene abundance, siRNAs are likely to also affect genes harbouring sequences partially complementary to the siRNAs, which will need to be further analyzed in order to exclude deleterious off-target effects. Another challenge will be to determine how to administer siRNAs specifically to the target cells in sufficient quantity. Recent studies have reported target tissue specific expression of siRNAs in mice as well as in non-human primates using viral vectors expressing short hairpin RNAs (shRNAs). Aptamer-shRNA chimaeras (18) may also be an interesting possibility to explore in order to deliver siRNAs specifically to certain cell types.
As a multitude of independent mutations (>800) have been described as causative of OI, it would be laborious to design separate allele specific siRNAs for each patient. We show that siRNAs differing for SNPs can be used to silence predominately one allele of COL1A2 in primary bone cells. The next step is to scan the full range of polymorphisms in the COL1A2 and COL1A1 and to design highly allele-specific siRNAs. By designing highly effective and specific siRNA pairs targeting each of two alleles of a particular SNP, rather than the actual mutation, the siRNA linked to the mutated allele could be used therapeutically in OI-patients heterozygous for this SNP. With a panel of siRNAs against common SNPs in the Collagen type I genes it would be possible to genotype the patient for common polymorphic positions and then advance with the most appropriate siRNA. As proof of principle, silencing of the T-allele of rs1800222 produced equally evident silencing when allele ratios were examined for a polymorphic position in exon 25 (rs 412777).
The results presented herein show that allele-preferential silencing of COL1A2 is possible in the desired target cells, and thus presents a framework for further efforts towards personalized RNAi therapy in OI.
Acknowledgements
We thank Anna-Lena Johansson for skilful technical assistance and Dr. Dominic Wright for his linguistic review. This work was supported by grants from the Swedish research council, project nr: 2007-2946.
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
References
1. Willing MC, Pruchno CJ, Atkinson M, Byers PH. Osteo-genesis imperfecta type I is commonly due to a COL1A1 null allele of type I collagen. Am J Hum Genet. 1992;51(3):508-15
2. Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, Hyland JC, Korkko J, Prockop DJ, De Paepe A, Coucke P, Symoens S, Glorieux FH, Roughley PJ, Lund AM, Kuurila-Svahn K, Hartikka H, Cohn DH, Krakow D, Mottes M, Schwarze U, Chen D, Yang K, Kuslich C, Troendle J, Dalgleish R, Byers PH. Consortium for osteogenesis imperfecta muta-tions in the helical domain of type I collagen: regions rich in le-thal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28(3):209-21
3. Rauch F, Glorieux FH. Bisphosphonate treatment in osteo-genesis imperfecta: which drug, for whom, for how long? Ann Med. 2005;37(4):295-302
4. Schwarze U, Hata R, McKusick VA, Shinkai H, Hoyme HE, Pyeritz RE, Byers PH. Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from muta-tions in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am J Hum Genet. 2004;74(5):917-30
5. Takeshita F, Ochiya T. Therapeutic potential of RNA inter-ference against cancer. Cancer Sci. 2006;97(8):689-96
6. Izant JG, Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984;36(4):1007-15
7. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806-11
8. Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286(5441):950-2
9. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494-8
10. Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Rohl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene si-lencing in non-human primates. Nature. 2006;441(7089):111-4
11. Dykxhoorn DM, Schlehuber LD, London IM, Lieberman J. Determinants of specific RNA interference-mediated silencing of human beta-globin alleles differing by a single nucleotide polymorphism. Proc Natl Acad Sci U S A. 2006;103(15):5953-8
12. Hickerson RP, Smith FJ, Reeves RE, Contag CH, Leake D, Leachman SA, Milstone LM, McLean WH, Kaspar RL. Sin-gle-nucleotide-specific siRNA targeting in a dominant-negative skin model. J Invest Dermatol. 2008;128(3):594-605
13. Schwarz DS, Ding H, Kennington L, Moore JT, Schelter J, Bur-chard J, Linsley PS, Aronin N, Xu Z, Zamore PD. Designing siRNA that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2006;2(9):e140
14. Millington-Ward S, McMahon HP, Allen D, Tuohy G, Kiang AS, Palfi A, Kenna PF, Humphries P, Farrar GJ. RNAi of COL1A1 in mesenchymal progenitor cells. Eur J Hum Genet. 2004;12(10):864-6
15. Wang Q, Marini JC. Antisense oligodeoxynucleotides selec-tively suppress expression of the mutant alpha 2(I) collagen al-lele in type IV osteogenesis imperfecta fibroblasts. A molecular approach to therapeutics of dominant negative disorders. J Clin Invest. 1996;97(2):448-54
16. Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, Ueda R, Saigo K. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 2004;32(3):936-48
17. Ge B, Gurd S, Gaudin T, Dore C, Lepage P, Harmsen E, Hudson TJ, Pastinen T. Survey of allelic expression using EST min-ing. Genome Res. 2005;15(11):1584-91
18. McNamara JO 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24(8):1005-15
Author contact
![]() Correspondence to: Östen Ljunggren, Department of Medical Sciences, Uppsala University Hospital, SE-751 85 Uppsala, Sweden. Tel: 018-611 49 06; Fax: 018-55 36 01; E-mail: Osten.Lunggrenuu.se
Correspondence to: Östen Ljunggren, Department of Medical Sciences, Uppsala University Hospital, SE-751 85 Uppsala, Sweden. Tel: 018-611 49 06; Fax: 018-55 36 01; E-mail: Osten.Lunggrenuu.se

 Global reach, higher impact
Global reach, higher impact