3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2008; 5(6):319-326. doi:10.7150/ijms.5.319 This issue Cite
Research Paper
Controlling Osteogenesis and Adipogenesis of Mesenchymal Stromal Cells by Regulating A Circadian Clock Protein with Laser Irradiation
1. Frontier Research Center, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871, Japan
2. PRESTO, Japan Science and Technology Agency, 4-1-8 Honcho Kawaguchi, Saitama, 332-0012, Japan
3. Division of Sustainable Energy and Environmental Engineering, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, Osaka, 565-0871, Japan
Received 2008-9-16; Accepted 2008-10-23; Published 2008-10-26
Abstract
Mesenchymal stromal cells (MSCs) are multipotent cells present in adult bone marrow that replicate as undifferentiated cells and can differentiate to lineages of mesenchymal tissues. Homeostatic control of bone remodelling maintains bone mass by insuring that bone resorption and bone formation occur sequentially and in a balanced manner. As most homeostatic functions occur in a circadian manner, a circadian clock could control bone mass. Here, we show that laser irradiation can direct the osteogenesis and adipogenesis of mouse MSCs by altering the intracellular localization of the circadian rhythm protein Cryptochrome 1 (mCRY1). After laser irradiation (wavelength: 405 nm) to MSCs, circadian rhythm protein, mCRY1 and mPER2, were immunostained and histochemical stainings for osteogenic or adipogenic differentiation were observed. Laser irradiation promoted osteogenesis and reduced adipogenesis of MSCs, induced the translocation of mCRY1 and mPER2 protein from the cytoplasm to the nucleus, and decreased mCRY1 mRNA levels quantified by real-time PCR. Since the timing of nuclear accumulation of clock proteins constitutes an important step in the transcription-translation feedback loop driving the circadian core oscillator, laser irradiation could provide a simple and effective technology for clock protein localization and turnover. Our results also indicate that mCRY1 is a master regulator of circadian rhythm that regulates the differentiation of MSCs. Laser irradiation could provide a simple and effective means of controlling the fate of MSCs as a therapeutic strategy and act 'molecular switch' of regulatory proteins by suppressing CRY transcription. Furthermore, this model system may be useful for exploring the crosstalk between circadian rhythm and cell differentiation.
Keywords: cryptochrome, osteogenesis, adipogenesis, laser, mesenchymal stromal cells
INTRODUCTION
Organisms have evolved various methods for efficient utilization of light energy. Light has an especially important role as a stimulus for many developmental processes. For example, blue light markedly affects growth and development of higher plants. These responses are mediated by a blue light photoreceptor, cryptochrome (CRY).1 CRY shares significant homology with Class I cyclobutane pyrimidine dimmers (CPD) photolyase, although it does not exhibit photolyase activity. CRY also binds flavin adenine dinucleotide (FAD),2,3 consistent with CRY mediating a light-dependent redox reaction similar to CPD photolyase. However, genetic analysis indicates that the CRYs, which utilize flavin as light-absorbing cofactors, are the primary circadian photoreceptors.4
Circadian rhythms are oscillations in the behaviour and biochemical reactions of organisms that occur with a periodicity of approximately twenty-four hours. Circadian rhythms are thought to confer a selective advantage to organisms by enabling them to pursue levels of activity that are optimal for growth and development and minimize susceptibility to predation and competition by establishing favourable temporal niches. In mammals, the core oscillator of the master circadian clock utilizes interacting positive and negative transcription-translation feedback loops.5 Proteins involved in these feedback loops include two cryptochrome genes, Cry1 and Cry2, three homologs of the period genes, Per1, Per2, and Per3, and the transcriptional activator genes, Clock and Bmal1 (Brain and Muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-Like protein 1).6 A key step in these feedback loops is the shutdown of CLOCK- and BMAL1-driven transcription by CRY proteins. BMAL1 and CLOCK contain two basic helix-loop-helix domains and bind E-box elements (CACGTG) in the Per and Cry clock genes to activate their transcription. This activity is a positive feedback loop of circadian rhythm regulation.7 The mammalian Period proteins (PER1 and PER2) and Cryptochrome proteins (CRY1 and CRY2) act as negative regulators of transcription driven by the BMAL1/CLOCK heterodimer. Therefore, E-box elements play a crucial role in homeostatic function.
For example, homeostatic control of bone remodelling maintains bone mass by insuring that bone resorption and bone formation occur sequentially and in a balanced manner.7,8 As most homeostatic functions occur in a circadian manner,9,10 a circadian clock could control bone mass11. Bone mass is regulated by osteoblasts that differentiate from mesenchymal stromal cells (MSCs). MSCs are multipotent cells that can replicate as undifferentiated cells and that have the potential to differentiate to lineages of mesenchymal tissues, including bone, cartilage, fat, tendon, and muscle.12,13 Accordingly, controlling the division and differentiation of MSCs would provide an exceptional therapeutic resource for the restoration of damaged or diseased tissue. However, several fundamental questions must be answered before it will be feasible to dictate the differentiation of MSCs implanted to mature organisms. In particular, a better understanding of how specific factors alter the differentiation of MSCs is essential. Here, we show that blue laser (wavelength; 405 nm) irradiation can induce and reduce the osteogenesis and adipogenesis by altering the intracellular localization of the circadian rhythm protein CRY1.
MATERIALS AND METHODS
Cell culture and laser irradiation
The mouse MSCs cell line (KUSA-A) was purchased from RIKEN Bioresource Center, Japan, and cultured in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal calf serum (FCS), 100 units/mL penicillin, and 0.1 mg/mL streptomycin at 37°C in a 5% CO2 atmosphere. For osteogenic induction, MSCs were seeded at 4×104 cells/well in a Black with Clear Bottom 96-well Microtest™ Optilux™ Plate (BD bioscience Inc., CA) for 12 hrs. Following the resetting of circadian rhythms by dexamethasone (100 nM for 1 hr),14,15 cells were irradiated with a blue laser (VLM 500®, Sumitomo Electric Industries, Ltd., Japan, wavelength: 405 nm, continuous wave) for 180 sec via a fiber attached to the bottom of the culture dish. The optical instrument with an automated stage for positioning was purchased from Sigma Koki Co., Ltd., Japan. The beam profile of this laser system was observed with a LEPAS-11 Laser Beam Profiler (Hamamatsu Photonics K.K., Japan). A diameter of circular beam was approximately 500 μm. A blue laser was irradiated to cells cultured only in the center of well. After laser irradiation, MSCs were incubated in osteogenic differentiation medium (DMEM supplemented with 10% FCS, 10 nM dexamethasone, 50 μg/ml ascorbic acid, and 2 mM β-glycerophosphate) or adipogenic differentiation medium (DMEM supplemented with 10% FCS, 100 nM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 10 μg/ml insulin, and 0.2 mM indomethacin) at 37°C in a 5% CO2 atmosphere for 5 days. As control experiments, MSCs were incubated in the same conditions above, except for laser irradiation.
Histochemical analysis
For immunostaining of mCRY1 or mPER2, cells were fixed with 4% formalin in phosphate buffered saline (PBS, pH 7.4) 24 hrs after irradiation. Fixed cells were incubated with a primary antibody directed against anti-mouse CRY1 (Alpha Diagnostic International, Inc., TX) or PER2 (Chemicon International, Inc., CA) for 1 hr at room temperature, followed by incubation with a Cy3-conjugated second antibody (Sigma-Aldrich, Inc. MO) for 1 hr at room temperature. Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI). Only irradiated cells (around 500μm in the center of plate well) were observed by fluorescence microscopy. To evaluate osteogenesis, MSCs were rinsed three times with PBS and fixed with 4% formalin in PBS. Fixed cells were incubated with a 1% Alizarin red-S (Sigma-Aldrich, Inc. MO) aqueous solution (pH 6.5) for 15 min at room temperature and then rinsed three times with PBS. For von Kossa staining, fixed cells were exposed to UV light for 30 min in the presence of 5% silver nitrate and then incubated with 5% sodium thiosulfate for 5 min. The stained cells were rinsed with deionized water. For ALP staining, cells were stained using naphthol AS-BI phosphate solution and fast red violet solution (ALP detection kit, Chemicon International, Inc., CA). For osteocalcin immunostaining, fixed cells were incubated with a primary antibody (LSL Co., Cosmo Bio, Japan) directed against mouse osteocalcin for 1 hr at room temperature, followed by incubation with a Cy3-conjugated second antibody (Sigma-Aldrich, Inc. MO) for 1 hr at room temperature. The calcium contents of differentiated cells were measured with the calcium-E test kit (Wako Pure Chemical Industries, Ltd., Japan). To examine the state of adipose differentiation, fixed cells were stained with 0.5% oil red O in an isopropanol/water solution for 1 hr. After staining, cultures were rinsed several times with a 70% ethanol solution.
RNA extraction and real time PCR
Total cellular RNA of laser irradiated or non-irradiated MSCs was extracted with the RNAqueous kit (Ambion, Inc., Japan) according to the manufacturer's instructions 24 hr after laser irradiation. All samples were subjected to DNase treatment to avoid DNA contamination. mCRY1 or Peroxisome Proliferator-Activated Receptor (PPAR) γ mRNA expression was quantified by real time PCR. Reactions were carried out using a Smart Cycler version II (Takara Bio, Inc., Japan) with a SYBR ExScript RT-PCR kit (Takara Bio, Inc., Japan). PCR was started with an initial incubation at 95°C for 15 min to activate the Taq DNA polymerase, then set at 94°C for 15 sec, 56°C for 30 sec, and 72°C for 30 sec, for 40 cycles. Fluorescent signals were measured at the end of each elongation step and the start points of their exponential curves were determined for conversion of the cycle number into the amount of PCR product. Purified cDNA was employed to generate standard curves. The PCR efficiency of the primer sets was checked to confirm that the dilution rate of the samples was not affected. First, the annealing temperature of the eight wells of the PCR reaction plate on the apparatus was changed linearly from 55°C to 65°C to determine the optimal annealing temperature between the two sets of PCR primers. After the final PCR step, the temperature was elevated to 95°C while monitoring the fluorescent signals in order to form the melting curves to check the specificity of the PCR amplification. The levels of mCRY1 mRNA were normalized to the amount of mouse ribosomal protein S18 (Rps18) mRNA in each sample. Values were calculated as means ± standard deviation (SD). Comparisons between groups were made using Student's t-test. Differences were accepted as significant when P < 0.01.
Statistical analysis
All the data were expressed as the mean ± the standard derivation of the mean. Statistical significance (defined as P values of less than 0.01) was evaluated based on the unpaired Student's t test (two-tailed).
RESULTS
Intracellular distribution of mCRY1 and mPER2, and real-time PCR quantification of mCRY1 mRNA after laser irradiation
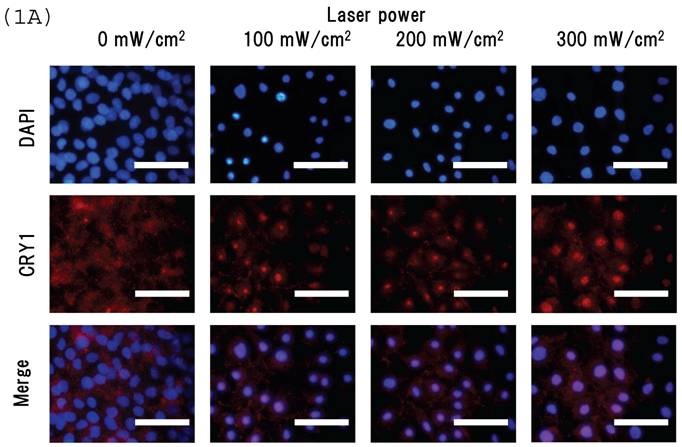
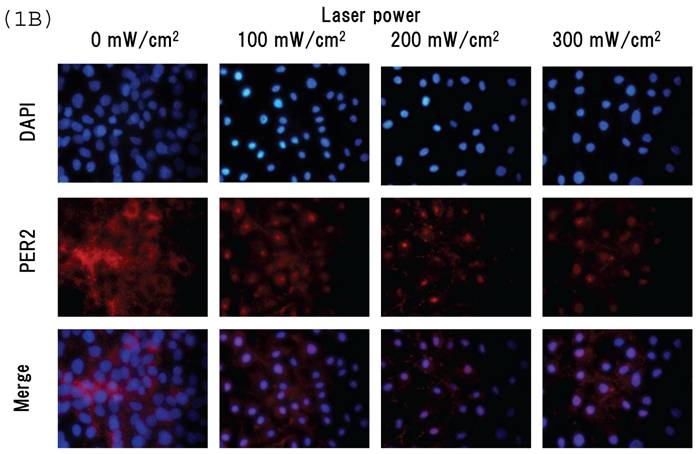
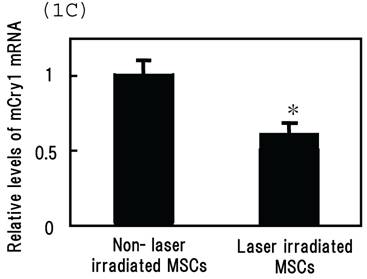
Intracellular distribution of mCRY1 and mPER2 after blue laser irradiation were shown in Figs. 1a and 1b. Blue laser irradiation to mouse MSCs promotes the nuclear accumulation of mCRY1 (Fig. 1A) and mPER2 (Fig. 1B). In addition, the mRNA levels of mCRY1, quantified by real-time PCR, decreased 24 hr after blue laser irradiation relative to non-irradiated cells (Fig. 1C). These results reveal that blue laser irradiation of mouse MSCs promotes the nuclear accumulation of mCRY1 and mPER2 and decreases their expressions via a negative feedback loop.
Intracellular location of (A) mCRY1 and (B) mPER2 proteins in MSCs 24 hr after laser irradiation. Cells were double-labeled with DAPI (blue, upper panel) and mCRY1 or mPER2 (red, center panel). The lower panel provides a merged image. mCRY1 and mPER2 localized to the cytoplasm prior to laser irradiation. After laser irradiation, proteins translocated to the nucleus. (C) mRNA levels of mCry1 in MSCs 24 hr after laser irradiation (100 mW/cm2) and in non-irradiated cells. Samples were normalized to mRps18. The mRNA levels of mCry1 decreased after blue laser irradiation relative to non-irradiated cells. Scale bars = 30µm *, p<0.01: significant difference between the relative mRNA levels of laser irradiated MSCs and controls.
Oteogenesis and adipogenesis of MSCs after laser irradiation
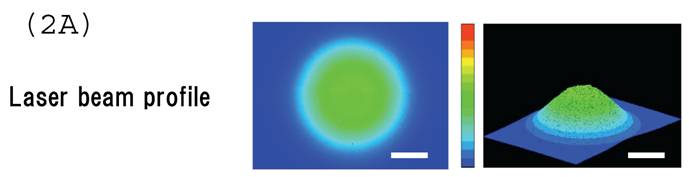
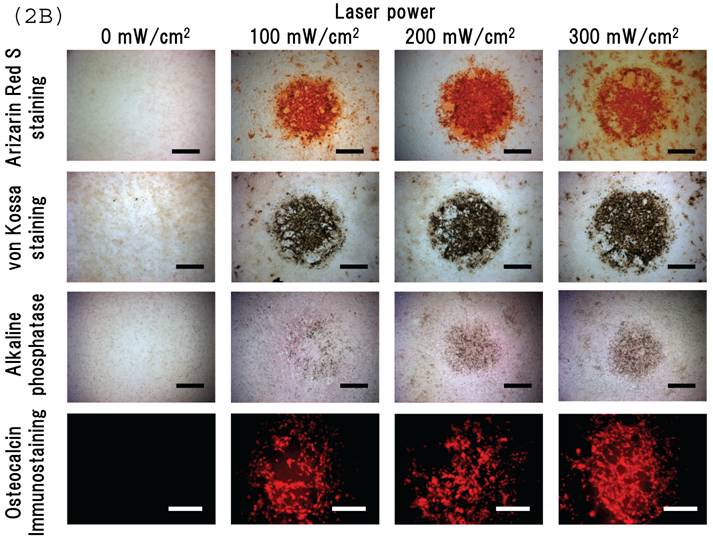
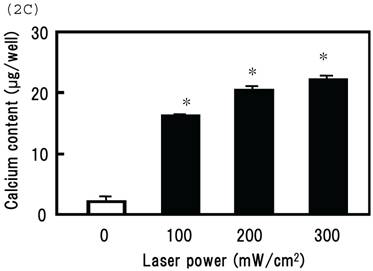
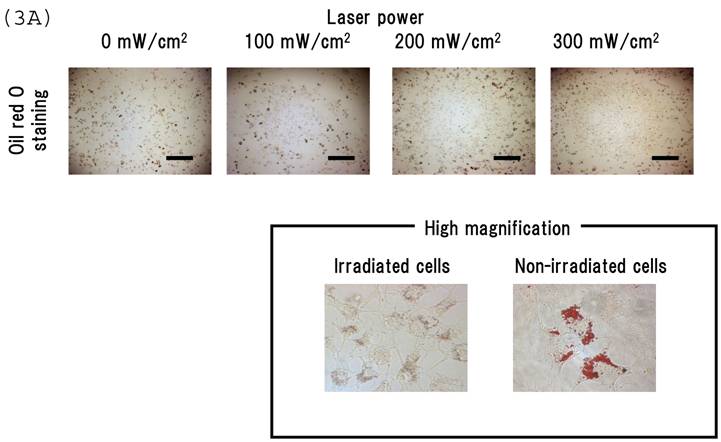
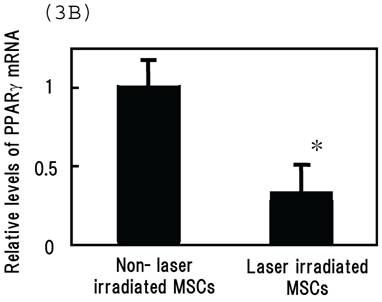
Irradiating cultured MSCs with a circular beam (Fig. 2A) stimulated osteogenesis exclusively within the area irradiated. Five days after blue laser irradiation, alizarin red-S and von Kossa staining revealed extensive calcium and calcium phosphate deposition that increased with laser energy (Fig. 2B). The calcium content of these wells was also increased (Fig. 2C). Laser irradiated samples displayed alkaline phosphatase (ALP) activity and were immunopositive for osteocalcin, a marker of osteoblast differentiation (Fig. 2B). Staining with oil red O (Fig. 3A) and PPARγ mRNA expression (Fig. 3B) indicated that blue laser irradiation decreased adipogenesis.
(A) The beam profile of the blue laser (wave length; 405 nm, continuous wave). MSCs were irradiated for 180 sec at various intensities. (B) Histochemical analysis of laser irradiated MSCs. Alizarin red-S staining of irradiated MSCs (magnification: x50). At 5 days post-irradiation, calcium deposition had increased around the cells in a dose-dependent manner. Calcium phosphate deposition was evaluated by von Kossa staining (magnification: x50). At 5 days after treatment, staining increased with increased laser energy. The area expressing alkaline phosphatase (ALP) activity was stained (magnification: x50). Laser irradiated samples displayed immunopositive staining for osteocalcin, a marker of osteoblast differentiation (magnification: x100). Scale bars = 200 (for Alizarin red-S, von Kossa, and ALA staining) and 100µm (for osteocalcin immunostaining). (C) The quantitative calcium content increased after blue laser irradiation relative to non-irradiated cells. Calcium content increases varied with laser energy. *, p<0.01: significant difference between the calcium content of laser-irradiated MSCs and controls.
(A) Staining with oil red O demonstrates that blue laser irradiation decreased adipogenesis relative to non-irradiated areas (magnification: x50). Higher magnification (x400) is shown in frame. Scale bars = 200 µm. As the nuclear localization of CRY proteins attenuates CLOCK- and BMAL1-driven transcription, laser irradiation may act 'molecular switch' for regulatory proteins by suppressing CRY transcription to limit the accumulation of lipid droplets in cells. (B) mRNA levels of PPARγ in MSCs 24 hr after laser irradiation (100 mW/cm2) and in non-irradiated cells. Samples were normalized to mRps18. The mRNA levels of PPARγ decreased after blue laser irradiation relative to non-irradiated cells. *, p<0.01: significant difference between the relative mRNA levels of laser irradiated MSCs and controls.
DISCUSSION
In this study, we demonstrate that blue laser irradiation of mouse MSCs promotes the nuclear accumulation of mCRY1 (Fig. 1A) and mPER2 (Fig. 1B). Since the timing of nuclear accumulation of clock proteins constitutes an important step in the transcription-translation feedback loop driving the circadian core oscillator,17 laser irradiation could provide a simple and effective technology for clock protein localization and turnover. In addition, transcriptional regulation is also fundamental to the circadian oscillations of clock gene expression. The mRNA levels of mCRY1 decreased after blue laser irradiation relative to non-irradiated cells (Fig. 1C). Protein products of CRY act together with PER proteins to inhibit CRY and PER transcription and close the autoregulatory feedback loop.5,6 These oscillations control output rhythms. The transcriptional feedback loop and a model of interlocked loops have been proposed as the basis for these oscillations. These results reveal that blue laser irradiation of mouse MSCs promotes the nuclear accumulation of mCRY1 and mPER2 and decreases their expressions via a negative feedback loop.
In addition, we demonstrate that irradiaton with a blue laser enhances the osteogenesis and suppress the adipgenesis of mouse MSCs. Irradiating cultured MSCs with a circular beam (Fig. 2A) stimulated osteogenesis exclusively within the area irradiated (Fig. 2B). Since a functional hallmark of osteoblasts is their ability to mineralize the extra cellular matrix (ECM), we stained irradiated cultures with alizarin red and von Kossa staining to detect calcium and calcium phosphate deposition, respectively. CRY is a key protein for the differentiation of MSCs and bone nodule formation. Mice lacking Cry1 and 2 (Cry1−/−;Cry2−/−) exhibit higher bone mass, consistent with the hypothesis that dysfunction of the molecular clock influences bone remodeling.11 In addition, the E-box is essential for tissue-specific transcriptional activation of mouse bone morphogentic protein (BMP)-4 and osteogenic lineage-specific novel transcriptional factor(s) recognizes this E-box.18 The diurnal variation in the synthesis of type I collagen and osteocalcin, the two main biosynthetic products of osteoblasts, supports this hypothesis.19,20
Adipocytes also play essential metabolic roles. They not only provide massive energy reserves but also secrete hormones and cytokines that regulate metabolic activities.21 The link between metabolic activity in adipocytes and circadian rhythm has been studied extensively. For example, glucose and lipid homeostasis exhibit circadian variation. More recently, the expression of adiponectin receptors in adipocytes has been reported to vary in a circadian fashion.22 Another clock protein, BMAL1, regulates adipogenesis and lipid metabolic activity in mature adipocytes.23 In our cultures, staining with oil red O and quantification of PPARγ mRNA indicated that blue laser irradiation decreased adipogenesis (Figs. 3A and 3B).
These phenomena were indicated that a blue laser irradiation is 'speeding up' a sequence of events that will occur anyway under these culture conditions. Thus, laser irradiation may act 'molecular switch' of regulatory proteins by suppressing CRY transcription to enhance the osteogensis and to limit the accumulation of lipid droplets in cells. Recent evidence indicates that at low-radiation doses light energy is absorbed by intracellular chromophores.24 Current models propose that low-level laser irradiation generates a small amount of singlet oxygen that influences the formation of adenosine triphosphate (ATP).25 In addition, laser irradiation may increase the transmembrane electrochemical proton gradient in mitochondria to improve the efficiency of the proton-motive force and generate greater calcium release by an antiport process.26 A number of different lasers with different wavelengths, including helium-neon (wavelength; 632.8 nm), gallium-aluminum-arsenide (wavelength; 805±25 nm), and gallium-arsenide (wave length; 904 nm), have been used at different intensities and treatment schedules for repairing bone defects. However, few studies have attempted to quantify the effect of low-level laser therapy on bone formation.27
We demonstrate that mCRY1 is a master regulator of circadian rhythm that can regulate the differentiation of MSCs following blue laser irradiation. The detailed mechanism of relationship between photo-acceptance of CRY, CRY down-regulation, and cell differentiation by blue laser irradiation were not unclear. However, irradiation with lasers at different wavelengths, 664 and 808 nm, did not alter the intracellular distribution of mCRY1 and mPER2 and did not affect on the cell differentiation (data not shown). This observation provides additional evidence for the specific photoreceptive function of CRY proteins. There are several reports that mCRY is the only blue-light photoreceptor implicated in circadian photoresponses.14,28-33 Mammalian CRY1 bound to FAD contributes to photoreception of 405 nm light by murine cells. Although the blue laser irradiation used in this study was extremely high energy compared with that in natural light, our results indicate that mCRY1 plays an important role in the control of the differentiation of MSCs. We propose that blue laser irradiation of MSCs could provide a simple and effective technology for clock protein localization and turnover as the timing of nuclear accumulation of clock proteins constitutes an important step in the transcription-translation feedback loop driving the circadian core oscillator. We examine the effect of laser irradiation in other types of cells, such as primary osteoblasts and primary bone marrow stromal cells. Those results will be reported in near future. As this technique could readily be applied in situ to control the differentiation of MSCs at an implanted site within the body, this approach may have therapeutic potential for the restoration of damaged or diseased tissue. Furthermore, these findings provide an excellent opportunity to gain insights into the cross-talk between circadian rhythms, bone formation and adipose metabolism.
Acknowledgements
A part of this paper was published as a patent, US-patent Pub. No. 20080057580 A1, Mar. 6, 2008.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
1. Short TW, Briggs WR. The transduction of blue light signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:143-171
2. Lin C, Robertson DE, Ahmad M, Raibekas AA, Jorns MS, Dutton PL, Cashmore AR. Association of flavin adenine dinucleotide with the Arabidopsis blue light receptor CRY1. Science. 1995;269:968-970
3. Todo T, Kim ST, Hitomi K, Otoshi E, Inui T, Morioka H, Kobayashi H, Ohtsuka E, Toh H, Ikenaga M. Flavin adenine dinucleotide as a chromophore of the Xenopus (6-4)photolyase. Nucleic Acids Res. 1997;25:764-768
4. Hsu DS, Zhao X, Zhao S, Kazantsev A, Wang RP, Todo T, Wei YF, Sancar A. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 1996;35:13871-13877
5. Young MW. Circadian rhythms. Marking time for a kingdom. Science. 2000;288:451-453
6. Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013-1019
7. Rodan GA Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508-1514
8. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342
9. Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407-441
10. Perreau-Lenz S, Pevet P, Buijs RM, Kalsbeek A. The biological clock: the bodyguard of temporal homeostasis. Chronobiol Int. 2004;21:1-25
11. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803-815
12. Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147
13. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147
14. Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signalling. Science. 2000;289:2344-2347
15. Wu X, Yu G, Parks H, Hebert T, Goh BC, Dietrich MA, Pelled G, Izadpanah R, Gazit D, Bunnell BA, Gimble JM. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42:861-870
16. Kobayashi K, Kanno S, Smit B, van der Horst GT, Takao M, Yasui A. Characterization of photolyase/blue-light receptor homologs in mouse and human cells. Nucleic Acids Res. 1998;26:5086-5092
17. Young MW, Kai SA. Time zones: a comparative genetics of circadian clocks. Nature Rev Genet. 2001;2:702-715
18. Kawasaki S, Ebara S, Nakayama K, Takaoka K. The E-Box motif, recognized by tissue-specific nuclear factor(s), is important for BMP-4 gene expression in osteogenic cells. Biochem Biophys Res Commun. 1999;263:560-565
19. Simmons DJ, Nichols G Jr. Diurnal periodicity in the metabolic activity of bone tissue. Am J Physiol. 1966;210:411-418
20. Gundberg CM, Markowitz ME, Mizruchi M, Rosen JF. Osteocalcin in human serum: a circadian rhythm. J Clin Endocrinol Metab. 1985;60:736-739
21. Spiegelman BM Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531-543
22. Bluher M, Fasshauer M, Kralisch S, Schon MR, Krohn K, Paschke R. Regulation of adiponectin receptor R1 and R2 gene expression in adipocytes of C57BL/6 mice. Biochem Biophys Res Commun. 2005;329:1127-1132
23. Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071-12076
24. Friedmann H, Lubart R, Laulicht I. A possible explanation of laser-induced stimulation and damage of cell cultures. J Photochem Photobiol B. 1991;11:87-91
25. Amat A, Rigau J, Waynant RW, Ilev IK, Tomas J, Anders JJ. Modification of the intrinsic fluorescence and the biochemical behavior of ATP after irradiation with visible and near-infrared laser light. J Photochem Photobiol B. 2005;81:26-32
26. Mochizuki-Oda N, Kataoka Y, Cui Y, Yamada H, Heya M, Awazu K. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett. 2002;323:207-210
27. Ozawa Y, Shimizu N, Kariya G, Abiko Y. Low-Energy Laser Irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone. 1998;22:347-354
28. Miyamoto Y, Sancar A. Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci U S A. 1998;95:6097-6102
29. Thresher RJ, Vitaterna MH, Miyamoto Y, Kazantsev A, Hsu DS, Petit C, Selby CP, Dawut L, Smithies O, Takahashi JS, Sancar A. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490-1494
30. van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH Yasui A. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627-630
31. Egan ES, Franklin TM, Hilderbrand-Chae MJ, McNeil GP, Roberts MA, Schroeder AJ, Zhang X, Jackson FR. An extraretinally expressed insect cryptochrome with similarity to the blue light photoreceptors of mammals and plants. J Neurosci. 1999;19:3665-3673
32. Devlin PF, Kay SA. Cryptochromes-bringing the blues to circadian rhythms. Trends Cell Biol. 1999;9:295-298
33. Thompson CL, Selby CP, Partch CL, Plante DT, Thresher RJ, Araujo F, Sancar A. Further evidence for the role of cryptochromes in retinohypothalamic photoreception/phototransduction. Brain Res Mol Brain Res. 2004;122:158-166
Author contact
![]() Correspondence to: Toshihiro Kushibiki, kushibikieng.osaka-u.ac.jp, Phone/Fax +81-6-6878-6330
Correspondence to: Toshihiro Kushibiki, kushibikieng.osaka-u.ac.jp, Phone/Fax +81-6-6878-6330

 Global reach, higher impact
Global reach, higher impact