Impact Factor
ISSN: 1449-1907
Int J Med Sci 2008; 5(3):113-120. doi:10.7150/ijms.5.113 This issue Cite
Research Paper
The relationship between the biochemical control outcomes and the quality of planning of high-dose rate brachytherapy as a boost to external beam radiotherapy for locally and locally advanced prostate cancer using the RTOG-ASTRO Phoenix definition
Radiation Oncology Department, Hospital A C Camargo, Sao Paulo, Brazil
Received 2008-3-31; Accepted 2008-6-3; Published 2008-6-4
Abstract
Purpose: To evaluated prognostic factors and impact of the quality of planning of high dose rate brachytherapy (HDR-BT) for patients with local or locally advanced prostate cancer treated with external beam radiotherapy (EBRT) and HDR-BT.
Methods and Materials: Between 1997 and 2005, 209 patients with biopsy proven prostate adenocarcinoma were treated with localized EBRT and HDR-BT at the Department of Radiation-Oncology, Hospital A. C. Camargo, Sao Paulo, Brazil. Patient's age, Gleason score (GS), clinical stage (CS), initial PSA (iPSA), risk group for biochemical failure (GR), doses of EBRT and HDR-BT, use of three-dimensional planning for HDR-BT (3DHDR) and the Biological Effective Dose (BED) were evaluated as prognostic factors for biochemical control (bC).
Results: Median age and median follow-up time were 68 and 5.3 years, respectively. Median EBRT and HDR-BT doses were 45 Gy and 20 Gy. The crude bC at 3.3 year was 94.2%. For the Low, intermediate and high risk patients the bC rates at 3.3 years were 91.5%, 90.2% and 88.5%, respectively. Overall survival (OS) and disease specific survival rates at 3.3 years were 97.8% and 98.4%, respectively. On univariate analysis the prognostic factors related bC were GR (p= 0.040), GS ≤ 6 (p= 0.002), total dose of HDR-BT ≥ 20 Gy (p< 0.001), 3DHDR (p< 0.001), BED-HDR ≥ 99 Gy1.5 (p<0.001) and BED-TT ≥ 185 (p<0.001). On multivariate analysis the statistical significant predictive factors related to bC were RG (p< 0.001), HDR-BT ≥ 20 Gy (p=0.008) and 3DHDR (p<0.001).
Conclusions: we observed that the bC rates correlates with the generally accepted risk factors described in the literature. Dose escalation, evaluated through the BED, and the quality of planning of HDR-BT are also important predictive factors when treating prostate cancer.
Keywords: high-dose rate brachytherapy, external beam radiotherapy, prostate cancer, RTOG-ASTRO Phoenix, biochemical failure, biochemical control
Introduction
World population median age is increasing as result of improvement in health care. With the emergence of prostatic specific antigen (PSA) screening, the proportion of cases of prostate cancer (PCa) diagnosed has been increasing. PCa is one of the most prevalent malignancies affecting men in the developed world. For male population of western countries, the probability of dyeing of PCa is about 3% [1]. The best management of both localized and locally advanced PCa remains controversial with a consensus that surgery, radiotherapy, hormonal therapy, and active surveillance can be used isolated or in combination to treat the different of risk groups (RG) for biochemical failure (BF) [2].
Significant clinical data are available demonstrating that patients treated with radiation therapy have a significantly better outcome as the dose to the prostate is increased [3-5]. There are also many published results demonstrating that conformal high dose rate brachytherapy (HDR-BT) is a successful method for delivering higher dose of radiation to the prostate [6,7]. HDR-BT is a very precise and conformal way of dose delivering comparable to three-dimensional conformal (3DRT) and intensity modulated (IMRT) external beam radiotherapy (EBRT) [8]. HDR-BT also has some potential additional advantages over normal tissues sparing and on reducing miss dose to the prostate, due imprecise target localization, treatment setup uncertainties, organ motion and or deformation during the treatments, with a relative low incidence of severe acute and late side effects [9-11]. In this study we evaluated the relationship between quality of planning and prognostic factors related to biochemical control (bC), according to the RTOG-ASTRO Phoenix Consensus Conference [12] and the quality of planning of HDR-BT.
Material and methods
This is a retrospective analysis performed to assess the efficacy of the use of three-dimensional (3DHDR) over bi-dimensional planning (2DHDR) of HDR-BT and EBRT in a population of patients with clinically localized or locally advanced PCa. This work on human beings complies with the principles laid down in the DECLARATION OF HELSINKI and has been approved by the ethical committee of Hospital A. C. Camargo, Sao Paulo, Brazil.
Patient Group
The charts of patients with local or locally advanced biopsy proven prostate adenocarcinoma treated at the Department of Radiation Oncology, Hospital A.C. Camargo, Sao Paulo, Brazil were retrospectively reviewed. Details as Gleason scored (GS), the initial PSA value, clinical stage (CS) using the 1992 AJCC clinical stage and use of 3D HDR-BT planning were collected.
Definition of risk group for biochemical failure
Patients were grouped into 3 different subgroups of risk for BF. The low risk (LR) group encompassed patients with CS T2a or lesser, GS less than 7 and initial PSA value equal or lesser than 10 ng/ml. Patients with either stage T2b, GS 7 or initial PSA value ranging from 10–20 ng/ml were considered intermediate risk (IR) for BF. The remaining patients or patients who presented two or more of the characteristics of the IR group were grouped into the high risk (HR) group for BF. At the discretion of the referral urologists, patients into any of the groups received a course of neoadjuvant androgen blockage (NAAD), with goserelin and or flutamide or ciproteron acetate, 3 to 6 months prior to EBRT. Patients
External beam radiotherapy (EBRT)
In the first three years of treatment conventional EBRT planning was used. In summary, patients had a pretreatment diagnostic computed tomography scan (CT), urethrogram and rectal contrast to assist in defining prostate, seminal vesicles and normal tissue volumes at risk. The prostate and seminal vesicles were irradiated through a 6 MV Varian linear accelerator, with four-field box technique and individual protections with cerrobend blocks. After 1999, all patients were treated with localized 3DRT.
High dose rate brachytherapy (HDR-BT)
The technique of HDR-BT has been previously published elsewhere [8]. The volume limitation for this approach is prostate volume less than 60 cc. In brief, the implant procedures were performed 10 to 15 days after the end of EBRT, with no prior pre-planning. At the beginning of the implant, a Foley catheter was inserted to help visualize the urethra. With the patient in lithotomic position and under spinal anesthesia, a TRUS probe from Siemens Sonoline Prima Ultrasound System (Siemens Medical Solutions–Ultrasound Division, Mountain View, CA) was positioned as parallel as possible to the prostatic urethra. At the first moment two metallic markers were inserted into the gland, one in the apex and the other in the base of the prostate, to ascertain quality control of any needle displacement during the treatment and to allow corrections whenever it was necessary. All the implants were performed with steel needles with 75% of the needles located on the periphery and 25% around the urethra. The tips of the needles were always kept below the bladder mucosa and out of the urethra. In the 2DHDR technique the apex and base of the gland were identified using transverse and sagittal TRUS images, which were also used for defining the active length of each needle inside the prostate. After the end of the implant a CT scan was performed to ensure that the entire gland volume was implanted, but semi-orthogonal X rays were used for planning and dosimetric calculations. Plans were generated with the Nucletron Planning System BPS V11.2 (Nucletron B.V., Veenendaal, The Netherlands). In reconstruction needles were digitized at both ends and at least three axial plans with 10-mm increment were generated. The dose prescription was in a point of minimum dose defined in the central plan of the implant. The treatment was optimized using the standard geometric optimization and median isodose line prescription was 85% (range 82–91%). Dose prescriptions were 4 or 5 Gy per fraction, b.i.d., up to 16 Gy for LR and 20 Gy for IR and HR patients. Treatment was delivered via the micro-Selectron-HDR Ir-192 remote afterloading system, source strength ranging from 1.84 to 4.61 cGy h-1 m-2 (4.5–10 Ci).
On August, 2000, we switched to 3D image guided CT based planning brachytherapy. The Siemens Sonoline Prima Ultrasound System (Siemens Medical Solutions–Ultrasound Division, Mountain View, CA) and a Winston-Barzell track-stepper unit (Barzell-Whitmore Maroon Bells, Sarasota, FL) with integrated needle guide were used for the implant procedure. After the end of insertion of the needles the TRUS probe was removed, legs were laid flat, and plain orthogonal pelvic radiographs were taken with needles in place to identify the metallic markers and ensure quality control.
The patients were moved directly after the implantations to a CT simulator were a planning pelvic CT was done. Three-millimeter-step and three-millimeter-thick CT slices were collected using a spiral CT and sent on-line via network to the BrachyVision Planning System (Varian Medical Systems, Palo Alto, CA). The clinical target volume (CTV) and critical organs including bladder, rectum, and urethra were then contoured. The following volumes were defined: the CTV - Clinical Target Volume - represented by the entire prostate, the Planning Target Volume- PTV- represented by an expansion of the CTV 3 mm in the lateral and anterior dimension for each CT axial image. The most cephalad and caudad axial plane containing the prostate were expanded 5 mm. No expansions were made for the posterior region, given a PTV approximately 6 mm larger in the lateral, 3mm in the anterior and 10 mm longer in the caudal-cephalic dimensions than the CTV. Implant catheters were digitized using the CT reconstruction and only dwell positions inside the PTV were activated. The outer most mucosa surface of bladder and rectum were contoured in all CT images. The urethra was defined by the outer surface of the Foley catheter. Only the urethral volume within the PTV was contoured. The 3DHDR dose optimization was based on inverse planning, with at least 95% of the PTV volume receiving 100% of prescribed dose (V100 ≥ 95% of prescribed dose) [13]. Dose prescriptions ranged from 4 to 6 Gy per fraction, b.i.d., up to 16 Gy for LR and 20 or 24 Gy for IR and HR patients, respectively.
After completion of treatment patients were seen in follow-up 1 month later and after that every 2-4 months for the first 24 months. Thereafter patients were seen in follow up every 6-12 months.
Calculation of Biologically Effective Doses (BED)
To facilitate comparison between different fractionation schedules discussed the biologically effective doses (BED) for PCa were calculated and the iso-effect model using the LQ was used. The α/β-value assumed for the PCa was of 1.5 Gy, which is supported by radiobiological analyses [14]. Two assumptions were made: 1) complete repair occurs between fractions, and 2) there is no time factor.
Statistical analysis
The bC were calculated as the interval from pathologic diagnosis of PCa to BF or dead due prostate cancer progression. Date of BF, a rise in 2 ng/ml after the nadir had been richen, was defined according to the RTOG-ASTRO Phoenix Consensus Conference [12]. Pearson chi-square and t tests were used to compare differences in categorical and continuous patient characteristics, respectively. For disease specific survival (DSS) calculations only patients who died due prostate cancer were censored. Survival data were generated using the Kaplan-Meier method, with log-rank test used to compare equality of survivor functions. The Cox proportional hazard model was used for multivariate analysis. The covariates in multivariate analysis were BED from EBRT and HDR-BT, use of tridimensional planning for HDR-BT and risk group for BF. These variables were entered into the analysis using a stepwise procedure. Once the best model was selected, group risk was entered into the logistic regression last, to isolate its impact as much as possible. Statistical tests were performed using SPSS 13.0 (SPSS, Chicago, IL).
Results
There were 234 patients treated with combination of EBRT and HDR-BT between 1997 and 2005 at the Department of Radiation Oncology, Hospital AC Camargo, Sao Paulo, Brazil. Fifteen patients were excluded from the analysis because of they were lost to follow up after treatment. Seven patients had whole pelvis EBRT and were also excluded from the analysis. Three patients did not reach a nadir value bellow 1.0 ng/ml after the end of treatment and a revision of their charts showed that they already had metastatic disease at the time of initial approach, being also excluded from this analysis. Median age of the remaining 209 patients was 68 years (range, 47-83 years). Median follow-up was 5.3 years (range, 2 –10). There were 75 (35.8%) of patients into LR, 66 (31.6%) into IR group and 68 (32.6%) into HR group. Characteristics of patients are shown in Table 1.
The dose of EBRT ranged from 36 to 54 Gy (median 45 Gy) given in 20 to 30 daily fractions of 1.8 or 2.0 Gy. The total EBRT treatment time ranged from 4 to 7 weeks (median 5 weeks). The HDR-BT was performed after one to two weeks of the completion of EBRT in 204 patients. Five patients started their treatment by HDR-BT and after that had a course of EBRT. The total treatment time ranged from 5 to 9 weeks (median 7 weeks). The dose of HDR-BT ranged from 16 to 24 Gy given in 4 fractions, BID in two days. Median HDR-BT dose was 20 Gy.
The BED was calculated assuming an α/β ratio of 1.5 for PCa. The median BED for EBRT (BED-RT) was 104 Gy1.5 (range 84-118.8 Gy1.5) and for HDR-BT (BED-HDR) was 76 Gy1.5 (range 58.7-120 Gy1.5). The total BED (BED-TT) ranged from 142.7 Gy1.5 to 236.7 Gy1.5 with a median of 185 Gy1.5 (s.d. 18.5).
Patients characteristics
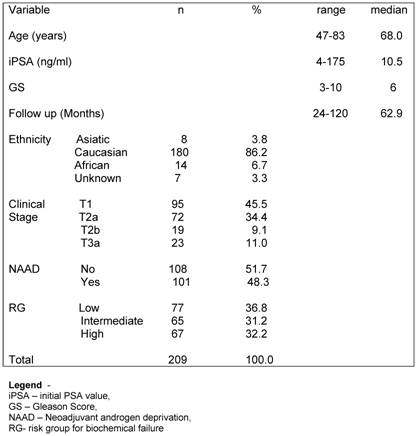
Overall survival (OS) rates at 3.3, 5 and 10 years were 97.8%m 95.7% and 90.6% respectively. The 3.3-, 5- and 10-year actuarial DSS rates were 98.4%, 97.7% and 95.5%, respectively, as shown in Figure 1. Eleven patients were dead at the time of this analysis. Five (2.4%) patients died due prostate cancer disease progression (local or distant) and 6 (2.9%) of other causes (3 of cardiologic related disease, 2 of a new second primary tumor and 1 of surgical complication after a salvage prostatectomy).
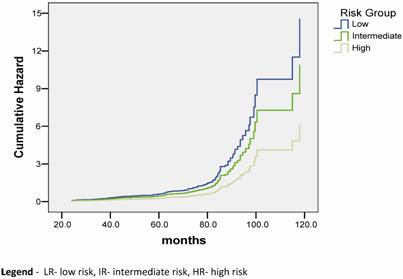
The crude bC rate at 3.3 year was 94.2%. For the LR, IR and HR the bC rates at 3.3 years were 91.5%, 90.2% and 88.5%, respectively, as shown in figure 2.
Disease specific survival.
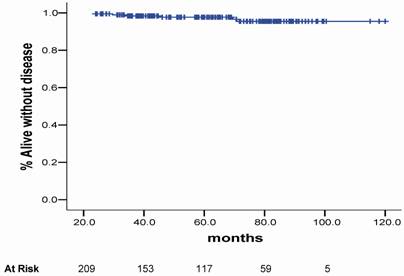
Biochemical control plots according to the risk group for BF.
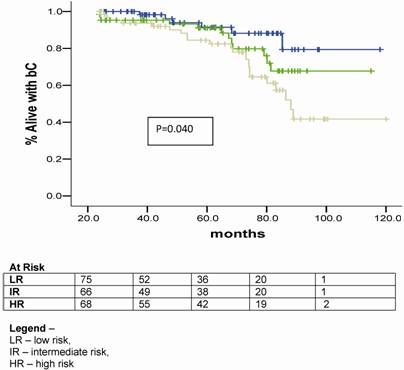
On univariate analysis the prognostic factors related bC were GR (p= 0.040), GS ≤ 6 (p= 0.002) and total dose of HDR-BT ≥ 20 Gy (p< 0.001). (Table 2)
Univariate analysis
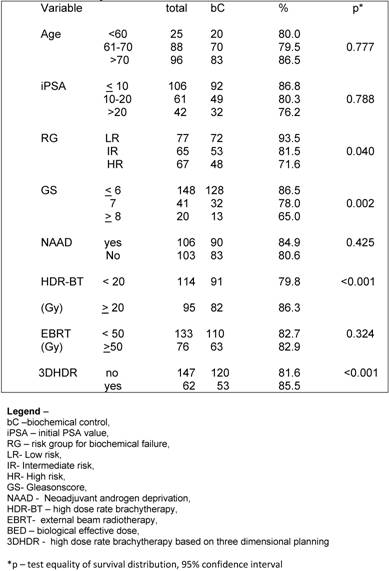
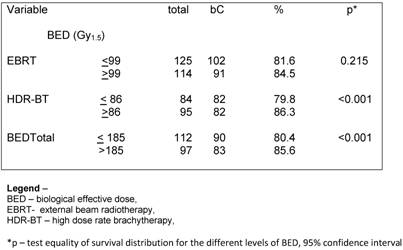
Regarding the BED, we observed that the BED-HDR ≥ 99 Gy1.5 and BED-TT (p<0.001) were statically significant perceptive factors related to bC instead of the BED-RT (p= 0.215) as shown in Table 3.
When evaluating the quality of planning we observed a survival advantage with 3DHDR (p< 0.001). (Figure 3).
On multivariate analysis the statistical significant predictive factors related to bC were RG p< 0.001 (CI – 1.147-3.561), HDR-BT ≥ 20 Gy p=0.008 (CI – 1.127-2.258) and 3DHDR p<0.001 (CI- 1.369-2.2696).
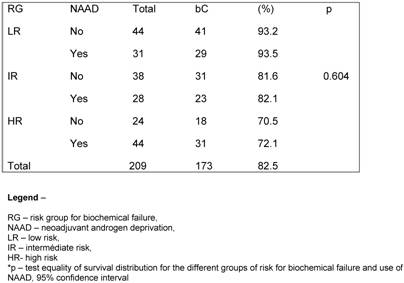
At the discretion of the referral urologists, patients into any of the groups received a course of NAAD. The population was well balanced in all groups with a minimum expected of 28 patients per subgroup. A hundred and six (50.7%) patients had no NAAD. When exploring the influence of NAAD for the different RG on bC, we observed no statically significant benefit of its in any group (p= 0.604). (Table 4).
Univariate analysis – bC according to BED
Influence of NAAD according to GR
Bichemical control plots acording to risk group for biochemical failure and stratified by quality of planning.

Hazard functions according to the risk group for BF.
Discussion
The main advantage of HDR-BT is its ability to deliver a relative high dose of radiation within a well-defined volume, with a rapid fall-off of dose outside the implanted area. This approach is ideal for the treatment of prostate cancer, where the gland lays very close to critical normal tissues, in particular the anterior rectal wall and bladder neck. Several retrospective studies with more than 5-year follow up have previously described the outcome of patients treated with combination of EBRT and HDR-BT [7, 15,16), but data from prospective randomized trail comparing results of this combination with dose escalation RT3D or IMRT is still missing.
To our knowledge, this is the first paper that explores the ability of 3DHDR on impacting in the final results. The use of 3DHDR was significantly related to the bC rates, in both uni and multivariate analysis (p< 0.001). Based in the use of 3D planning Martin et al. described this same strategy to enable real time distribution of the isodose in all CT slices, observing an increase accuracy of the delivery of the prescribed dose.
There is clinical evidence that suggests that prostate tumors contain a low proportion of proliferating cells, having a lower α/β ratio than the other epithelial tumors, in the range of 1.2–2.5 [17, 18]. We assumed, for BED calculation purposes a α/β ratio of 1.5 Gy for tumor and no correction for tumor repopulation in the interval between fractions was performed. The BED was calculated assuming a α/β ratio of 1.5 for PCa. The median BED for EBRT (BED-RT) was 104 Gy1.5 (range 84-118.8 Gy1.5) and for HDR-BT (BED-HDR) was 76 Gy1.5 (range 58.7-120 Gy1.5). The total BED (BED-TT) ranged from 142.7 Gy1.5 to 236.7 Gy1.5 with a median of 180 Gy1.5 (s.d. 18.5).
The total BED (BED-TT) ranged from 142.7 Gy1.5 to 236.7 Gy1.5. We observed a trend toward to a better bC rate for all risk groups when a BED-TT higher than 185 Gy1.5 was administered (p< 0.001). Similarly, a study by Tang et al. [19] evaluated results of 88 patients treated with EBRT alone (66 Gy) and EBRT (46 Gy) combined to HDR-BT (16 Gy to 20 Gy), the same dose levels e observed in this study, but with no NAAD was allowed in that group of patients. For the HDR-BT cohort, the overall actuarial 5-year bC was 67.4%. They noted a significant advantage (p= 0.011) when BED calculations were used to compare results of HDR-BT associated to EBRT. They have also compared the results in terms of HDR-BT total dose, 16 Gy versus 20 Gy, observing that the 5-year bC rates, using the RTOG-ASTRO Phoenix definition, were 58.8% and 77.3%, respectively (p= 0.07). In our analysis the bC rates had a statistical significant difference when total HDR-BT doses administered were < 20 Gy (79.8%) and ≥ 20Gy (86.3%), p< 0.001. We can also observe that the dose level of 66 Gy given in 33 fractions has a considerable inferior BED value than the same nominal total dose given by 46 Gy in 23 fractions and 20 Gy given in 4 fractions of HDR-BT. Our results in terms of dose levels of HDR-BT for the different RG have shown an improvement in bC with HDR dose escalation, with a trend toward better bC rates when BED-TT dose over 180 Gy1.5 was administered (p< 0.001).
Galalae et al [20] also investigated the long-term outcome by GR using HDR-BT and EBRT with or without NAAD. There were 611 patients grouped as follows: 46 patients into LR, 188 patients into IR and 359 patients considered HR. Using the ASTRO definition for BF they observed that the actuarial bC and disease free survival rates at 5-year and 10-year were 77%, 67%, 73% and 49%, respectively. For the different RG the actuarial 5-year bC rates were 96% for LR, 88% for IR and 69% for HR. They observed that GS and GR were statistical significant predictive factors of bC, what we also confirmed in the present study, were GS (p= 0.002) and RG (p= 0,040) were statistical significant predictive factors for BF. They observed that CS and iPSA were also statistical significant predictive factors, what was not confirmed in this study.
Deger et al. [21] evaluated 422 patients with localized prostate cancer treated between 1992 and 2001 with HDR-BT and 3DRT. All patients underwent laparoscopic pelvic lymph node dissection to exclude patients with lymphatic involvement. The BF was also defined according to the ASTRO criteria. The bC according to RG were 100% T1, 75% for T2 and 60% T3 at 5 years. Five-year bC were 81% in the LR, 65% in the IR and 59% in the HR. Five-year OS and bC were 87% and 94%, respectively. They also observed that iPSA, RG and age were significantly related to bC. In our study the use of NAAD was not associated with better bC (p= 0.425). When the patients were grouped according to the of BF and use of NAAD, there was not a statistical significance for a better bC related to the its use (p=0.604).
There are a scarce number of papers that have published the results of HDR-BT and EBRT for PCa using the RTOG-ASTRO Phoenix definition. This definition states that “To avoid the artifacts resulting from short follow-up, the reported date of control should be listed as 2 years short of the median follow-up” [12]. Chin et al. [22] published the results of 65 consecutive patients treated between 1998 and 2004 with combination of EBRT and HDR-BT given in 2 fractions. Sixty patients (92.3%) were considered IR or HR. With a median follow-up of 3.5 years (range 0.6-5.8), two patients had died of metastatic disease and other four patients had BF, giving a 3-year actuarial bC rate of 90.8%. Yamada et al. [23] also reported the results of 105 patients consecutively treated between 1998 and 2004 with EBRT (45-50.4 Gy) and HDR-BT (5.5-7.0 Gy per fraction). With a median follow-up of 44 months (8-79 months), the actuarial 5-year bC rates for LR, IR and HR were 100%, 98%, and 92%, respectively. In the current study the actuarial bC rates at 5-year were 91.8% for LR group, 79.3% for IR group and 69.1% for HR group (p= 0.040), respectively.
Patients considered HR have more chances of BF. This phenomenon could be a consequence of current inadequate imaging of lymph node or bone metastasis or due subclinical metastatic spread that remains undetectable during radical treatment. However, tumor biology itself could lead to the progression of the disease in the HR group. As a consequence, risk-adapted therapy is very important in these cases. The combination of EBRT and HDR-BT is an alternative strategy of dose escalation that can potentially achieve an even higher BED given to the tumour when compared 3DRT or IMRT, but for patients at HR the localized dose given by HDR-BT may be a potential disadvantage, because a microscopic spread outside the prostate and even its capsule may exist. In these cases, the combination of HDR-BT and EBRT can provide treatment to potential areas of microscopic spread. What is still not answered is if adding pelvic radiation, instead to localized EBRT in combination to HDR-BT for PCa patients with a more than 15% risk of positive lymph nodes will really improve outcome, and is stil controversial in literature [24].
A hundred and six (50.7%) patients had no NAAD in our analysis. Despite being a retrospective analysis, the patients were well balanced when having or not NAAD into the RG as seem in table 2. The use of NAAD is also still controversial for patients with IR to HR. Martinez et al. [25] in a study of 1,260 patients treated with pelvic EBRT and HDR-BT observed similar OS, DFS and bC for patients who were treated with or without the addition of a course of NAAD up to 6 months prior to radiation. They observed that NAAD did not confer a therapeutic advantage, and instead of that added side effects and cost. Furthermore, for the most unfavourable group, there was a higher rate of distant metastasis and more prostate cancer-related deaths. We could not observe a statistical significant benefit on BC rates with the use of NAAD in none of the GR.
Vargas et al. [26] performed a matched-pair analysis of patients treated with combined EBRT and HDR-BT from January 1993 to March 2003. A total of 1432 were evaluated. There were 755 cases identified as having a risk of pelvic limphonodes positive of more than 15% using the Roach formula. Of these, 255 cases were treated without pelvic EBRT and randomly matched by GS, CS and iPSA to 500 cases treated with pelvic EBRT, resulting in 250 pairs. As results they observed that BF, and OS were not significantly different for patients treated with pelvic radiotherapy. At a median follow-up of 4 years the bC and OS rates were 78%, 86%, 89% and 88%, respectively. In our analysis, the actuarial 5- and 10-year OS were 95.7% and 90.6%, while the actuarial bC rates at 5- and 10-year for the different RG were 91.8% and 82.3% for LR, 79.3% and 67.7% for IR, and 68.5% and 41.3% for HR (p= 0.040), respectively.
Our results and others suggested that bC is related not just clinical characteristics of patients, as RG and GS, but it is also related to treatment parameters as total dose of HDR-BT and BED. To our knowledge, this is the first paper that found a positive relationship between the quality of planning and the outcome.
So far in our own experience, HDR-BT associated to EBRT is a successful form of treatment of PCa, with results comparable to published data, results that tend to be even better with the introduction of image guided brachytherapy.
In conclusion, dose escalation, evaluated through BED, and 3DHDR are predictive factors for bC, as well as other already accepted factors described in literature as GS and RG. The challenges for the future are to determine which treatment option will have the best result for each patient and the role of NAAD when using EBRT combined to HDR-BT for IR and HR patients.
Conflict of Interest
The authors disclose any financial and personal relationships with people or organizations that could inappropriately influence this work.
References
1. Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56:11-25
2. Ali AS, Hamdy FC. The spectrum of prostate cancer care: from curative intent to palliation. Curr Urol Rep. 2007;8:245-52
3. Khoo VS. Radiotherapeutic techniques for prostate cancer, dose escalation and brachytherapy. Clin Oncol (R Coll Radiol ). 2005;17:560-571
4. Morgan PB, Hanlon AL, Horwitz EM, Buyyounouski MK, Uzzo RG, Pollack A. Radiation dose and late failures in prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1074-81
5. Symon Z, Griffith KA, McLaughlin PW, Sullivan M, Sandler HM. Dose escalation for localized prostate cancer: substantial benefit observed with 3D conformal therapy. Int J Radiat Oncol Biol Phys. 2003;57:384-90
6. Vargas CE, Martinez AA, Boike TP, Spencer W, Goldstein N, Gustafson GS, Krauss DJ, Gonzalez J. High-dose irradiation for prostate cancer via a high-dose-rate brachytherapy boost: results of a phase I to II study. Int J Radiat Oncol Biol Phys. 2006;66:416-23
7. Demanes DJ, Rodriguez RR, Schour L, Brandt D, Altieri G. High-dose-rate intensity-modulated brachytherapy with external beam radiotherapy for prostate cancer: California endocurietherapy's 10-year results. Int J Radiat Oncol Biol Phys. 2005;61:1306-16
8. Pellizzon AC, Nadalin W, Salvajoli JV, Fogaroli RC, Novaes PE, Maia MA, Ferrigno R. Results of high dose rate afterloading brachytherapy boost to conventional external beam radiation therapy for initial and locally advanced prostate cancer. Radiother Oncol. 2003;66:167-72
9. Pellizzon AC, Salvajoli JV, Maia MA, Ferrigno R, Novaes PE, Fogarolli RC, Pellizzon RJ. Late urinary morbidity with high dose prostate brachytherapy as a boost to conventional external beam radiation therapy for local and locally advanced prostate cancer. J Urol. 2004;171:1105-8
10. Pinkawa M, Fischedick K, Treusacher P, Asadpour B, Gagel B, Piroth MD, Borchers H, Jakse G, Eble MJ. Dose-volume impact in high-dose-rate Iridium-192 brachytherapy as a boost to external beam radiotherapy for localized prostate cancer--a phase II study. Radiother Oncol. 2006;78:41-46
11. Soumarova R, Homola L, Stursa M, Perkova H. Acute toxicity of conformal high dose interstitial brachytherapy boost in prostate cancer. Neoplasma. 2006;53:410-417
12. Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, Sandler H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965-74
13. Dannenberg T, Strassmann G, Vogt HG, Heyd R, Rogge B, Baltas D, Kurek R, Tunn U, Zamboglou N. New interstitial HDR brachytherapy technique for prostate cancer: CT based 3D planning after transrectal implantation. Radiother Oncol. 1999;52:257-260
14. Brenner D.J, Martinez A.A, Edmundson G.K, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio) similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6-13
15. Phan TP, Syed AM, Puthawala A, Sharma A, Khan F. High dose rate brachytherapy as a boost for the treatment of localized prostate cancer. J Urol. 2007;177:123-27
16. Galalae RM, Kovács G, Schultze J, Loch T, Rzehak P, Wilhelm R, Bertermann H, Buschbeck B, Kohr P, Kimmig B. Long-term outcome after elective irradiation of the pelvic lymphatics and local dose escalation using high-dose-rate brachytherapy for locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52(1):81-90
17. Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265-76
18. Bentzen SM, Ritter MA. The alpha/beta ratio for prostate cancer: what is it, really? Radiother Oncol. 2005;76:1-3
19. Tang JI, Williams SG, Tai KH, Dean J, Duchesne GM. A prospective dose escalation trial of high-dose-rate brachytherapy boost for prostate cancer: Evidence of hypofractionation efficacy? Brachytherapy. 2006;5:256-61
20. Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N, Eulau S, Gustafson G, Gribble M, Kovács G. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:1048-55
21. Deger S, Boehmer D, Roigas J, Schink T, Wernecke KD, Wiegel T, Hinkelbein W, Budach V, Loening SA. High dose rate (HDR) brachytherapy with conformal radiation therapy for localized prostate cancer. Eur Urol. 2005;47:441-48
22. Chin YS, Bullard J, Bryant L, Bownes P, Ostler P, Hoskin PJ. High dose rate iridium-192 brachytherapy as a component of radical radiotherapy for the treatment of localised prostate cancer. Clin Oncol (R Coll Radiol ). 2006;18:474-79
23. Yamada Y, Bhatia S, Zaider M, Cohen G, Donat M, Eastham J, Rabbani F, Schupak K, Lee J, Mueller B, Zelefsky MJ. Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy. 2006;5:157-64
24. Hong TS, Tome WA, Jaradat H, Raisbeck BM, Ritter MA. Pelvic nodal dose escalation with prostate hypofractionation using conformal avoidance defined (H-CAD) intensity modulated radiation therapy. Acta Oncol. 2006;45:717-27
25. Martinez AA, Demanes DJ, Galalae R, Vargas C, Bertermann H, Rodriguez R, Gustafson G, Altieri G, Gonzalez J. Lack of benefit from a short course of androgen deprivation for unfavorable prostate cancer patients treated with an accelerated hypofractionated regime. Int J Radiat Oncol Biol Phys. 2005;62:1322-31
26. Vargas CE, Demanes J, Boike TP, Barnaba MC, Skoolisariyaporn P, Schour L, Gustafson GS, Gonzalez J, Martinez AA. Matched-pair analysis of prostate cancer patients with a high risk of positive pelvic lymph nodes treated with and without pelvic RT and high-dose radiation using high dose rate brachytherapy. Am J Clin Oncol. 2006;29:451-57
Author contact
![]() Correspondence to: Antonio Cassio Assis Pellizzon, MD, Rua Prof Antonio prudente 211, Liberdade, Sao Paulo, SP - Brazil Cep 01509-020. Phones: +55-11-21895104/05; Fax: +55-11-21895101; Email: cassiopellizzoncom
Correspondence to: Antonio Cassio Assis Pellizzon, MD, Rua Prof Antonio prudente 211, Liberdade, Sao Paulo, SP - Brazil Cep 01509-020. Phones: +55-11-21895104/05; Fax: +55-11-21895101; Email: cassiopellizzoncom

 Global reach, higher impact
Global reach, higher impact