3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2007; 4(4):203-208. doi:10.7150/ijms.4.203 This issue Cite
Research Paper
In vivo bactericidal activities of Japanese rice-fluid against H. pylori in a Mongolian gerbil model
1. Department of Surgery, Shinshu University School of Medicine, Matsumoto, Japan
2. Agricultural Technology Institute, Nagano Farmer's Federation, Japan
3. Nagano Kohno, Co., Ltd., Japan
4. Department of Biomedical Laboratory Sciences, School of Health Sciences, Shinshu University School of Medicine, Japan
Received 2007-6-11; Accepted 2007-8-9; Published 2007-8-10
Abstract
Purpose: The antibiotic effect of rice-fluid on Helicobacter pylori infection was investigated using a Mongolian gerbil model.
Methods: Gerbils were divided into four groups: H. pylori -infected, rice-fluid-treated animals (group A); H. pylori -infected, untreated animals (group B); uninfected, rice-fluid-treated animals (group C); and uninfected, untreated animals (group D). Group A and B animals were killed 14 weeks after H. pylori infection and group C and D animals were killed at the same age. The stomachs were examined for histology, 5'-bromo-2'-deoxyuridine (BrdU) labeling, and the bacterial burden. Serum anti-H. pylori antibody titers were also tested.
Results: The positive incidence of H. pylori -culture was 25 and 84 % in groups A and B, respectively (p<0.01). Both the degree of inflammation and the BrdU labeling index in group A were significantly lower than those in group B.
Conclusions: Rice-fluid showed an antibiotic effect on H. pylori and an anti-inflammatory effect on the H. pylori -associated gastritis.
Keywords: Japanese Rice-fluid, Helicobacter pylori, Mongolian gerbil, eradication, prevention
1. Introduction
Helicobacter pylori is the most important etiological agent of chronic gastritis and peptic ulcer and it is also known to increase the risk of gastric cancer [1-4]. The Mongolian gerbil is a useful animal model to investigate human gastric disorders: H. pylori easily infects the stomach of Mongolian gerbils, thereby inducing human-mimicking gastritis, peptic ulcer and intestinal metaplasia in the stomach. In addition, this animal model can develop gastric adenocarcinoma by inoculation of H. pylori [5].
Accumulating evidence has demonstrated that the eradication of H. pylori in the stomachs by the administration of oral antimicrobial agents results in the resolution of H. pylori-associated gastroduodenal diseases [6-8]. In addition, the eradication of H. pylori is also reported to decrease the risk of gastric carcinogenesis in Mongolian gerbils [9, 10]. Triple combination therapy, using two antibacterial antibiotics and a proton pump inhibitor, achieved a high eradication rate [11-13]. However, such combination therapy does not always successfully eradicate H. pylori. In recent years, the increased occurrence of clarithromycin- and/or metronidazole-resistant strains of H. pylori has become a problem [14, 15]. In addition, antibiotics cannot be used due to drug allergies in some patients and the antibiotic therapy is also occasionally associated with adverse events. Furthermore, it is considered to be inappropriate to administer antimicrobial agents long-term to prevent H. pylori infection. Consequently, it is important to investigate the use of non-antibiotic therapies for H. pylori infection, which are highly effective and safe.
Many investigators have researched non-antibiotic therapies for H. pylori infection [16-21], utilizing plant extracts and constituents, probiotics, antioxidants, and so on. However, regarding rice-extract, no studies have yet been reported except ours [22]. In our previous study using an animal model, a Rice Power Extract (RPE) showed a suppressive effect on gastric mucosal inflammation, but it did not show any significant decrease in the viable H. pylori obtained from stomach samples [22]. This time, we employed a different type of rice extract, Japanese-Rice-Fluid (JRF, patent number 3655880). The JRF has already been reported to show a strong in vitro bactericidal activity against H. pylori [23] and there were no resistant strains of H. pylori [23]. In addition, it is easily manufactured and has recently been adopted as an ingredient in several kinds of liquid food as the main supplemental ingredient, and it is commercially available all over Japan.
In this study, we investigated the antibiotic effect of JRF on H. pylori infection in vivo and its anti-inflammatory effects on the H. pylori-induced gastritis, using a Mongolian gerbil model.
2. Materials and Methods
Animals
Specific pathogen-free male Mongolian gerbils (Meriones unguiculatus) (MGS/Sea) (Seac Yoshitomi, Ltd., Fukuoka, JAPAN), 7 weeks old, were housed in plastic cages with hard wood chips in an air-conditioned biohazard room for infection with a 12 hours light-12 hours dark cycle. They were given food (CE-2, Clea Japan Inc., Tokyo, Japan) and water or the JRF as drinking water in bottles ad libitum. Approval for this study was obtained prior to experimentation from the animal ethics committee, and all procedures were performed in compliance with Guidelines for the Care and Use of Laboratory Animals in Shinshu University.
Bacterial inoculation
H. pylori (ATCC43504, CagA+, VacA+, American Type Culture Collection, Rockville, MD, USA) was grown in Brucella broth (Becton Dickinson, Cockeysville, MD, USA) containing 10 % v/v horse serum at 37 °C under microaerophilic conditions (15% CO2) at high humidity for 40 hours with gentle shaking (150 rpm). After each gerbil had fasted for 24 hours, samples containing 1x 10 colony-forming units (CFU) per milliliter (0.8 ml) were used as the inoculum, which were delivered via an oral catheter.
Chemicals
The production of the Japanese rice-fluid (JRF) was conducted in 5 phases (patent number 3655880). Phase 1 involved the addition of 15-times volume of distilled water to polished Japanese raw rice. Phase 2 involved their incubation at 128 °C under pressure of 0.1 to 0.5 Mpa for 5 min. In phase 3, they were completed mixed by homogenizing, and then were cooled down to 40 to 55 °C. In phase 4, they were treated for 15 to 30 min. with the addition of some proteinase and amylase complexes, including alpha-amylase, beta-amylase, and gluco-amylase. In the last phase 5, the added enzymes were inactivated by heating the mixture up to 95 °C. Based on our preliminary experiment, the JRF containing 25% saccharides was used in this study. The JRF also contains 1 % protein, but no lipid.
Experimental protocol
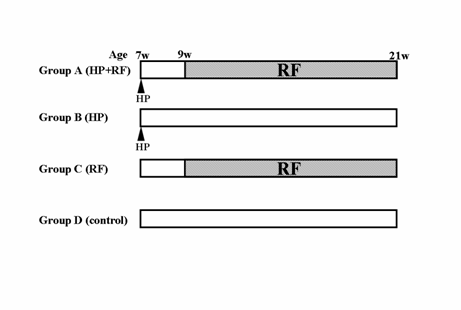
Fifty gerbils were divided into 4 groups. Group A comprised 20 gerbils that were inoculated with H. pylori at 7 weeks of age and given JRF as drinking water in bottle ad libitum from 2 weeks until 14 weeks after H. pylori inoculation. Group B comprised 19 gerbils that were inoculated with H. pylori and given autoclaved distilled water. Group C (6 gerbils) and D (5 gerbils) were not inoculated with H. pylori; Group C was given JRF and Group D was given distilled water. Following a 24-h fasting period, with free access to drinking water without JRF, the gerbils were sacrificed. All gerbils were sacrificed under deep ether anesthesia at 21 weeks of age and then their stomachs were excised. Thirty minutes before being sacrificed, the gerbils were given 5'-bromo-2'deoxyuridine (BrdU) intraperitoneally at a dose of 200 mg/Kg. The excised stomachs were cut open along the greater curvature and then were divided in half along the lesser curvature. One half was used for a culture study while the other was used for histopathological analyses. (Fig. 1)
Experimental protocol. HP: Inoculation of Helicobacter pylori. w: weeks. The rice-fluid (RF) was given ad libitum from 9 weeks of age to the end of the experiment. All gerbils were sacrificed 14 weeks after inoculation of HP (21 weeks of age).
Bacterial cultures
The excised stomachs (half) were homogenized with 5 ml of Brucella broth and then the resulting samples were diluted serially, from 1:10 to 1:1000000, with Brucella broth for the culture of H. pylori. Aliquots (100 ul) of the dilutions were inoculated on to H. pylori agar plates (Nissui Pharmaceutical Co, Tokyo, Japan). All plates were incubated at 35 °C for 7 days under microaerophilic conditions and high humidity. The density of H. pylori was assessed as CFU per whole stomach.
Serology
Anti-H. pylori IgG values in sera from gerbils were determined by ELISA developed in our laboratory [4,24]. The reference serum, which was pooled from sera of anti-H. pylori IgG-positive gerbils, was diluted serially from 1:100 to 1:3,200 with PBS (pH 7.4) containing 4% bovine serum albumin, and the amount of anti-H. pylori IgG corresponding to a 1:3,200 dilution was expressed as a reference value of a 1.0 arbitrary index (AI). Microwell strips coated with H. pylori antigens from a GAP-IgG kit (Biomerica, Newport Beach, Calif.) were used. The antigens in the GAP-IgG kit were acid extracts of organisms derived from the H. pylori ATCC 43504 strain. Aliquots of 100 µl of reference serum or 1:200 of diluted serum were added to the wells, and the plates were incubated for 1 h at room temperature. After washing was done, 100 µl of HRP-conjugated anti-gerbil IgG (diluted 1:1,500 in PBS containing 0.05% Tween 20 [PBS-T]) was added, and the plates were incubated for 30 min at room temperature. The plates were washed and incubated with 100 µl of substrate (0.35 mg of 3,3',5,5'-tetramethylbenzidine per ml and 0.15 mg of H2O2 per ml) for 10 min. After stopping the reaction with 1 N HCl, the color was read at 450 nm. The serum anti-H. pylori IgG value was determined from a standard curve of calibrators.
Histopathological analyses
The excised stomachs (half) were fixed in 20 % phosphate-buffered formalin for 24 hours at 4℃ and then were cut along the longitudinal axis into five strips, which were then embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (HE) for morphological observations, and with alcian blue (pH 2.5)-periodic acid Schiff (PAS) for the detection of mucin-containing cells. Because the inflammatory component of the mucosa was virtually identical to that found in human H. pylori gastritis, the degree of inflammation could be graded according to the Updated Sydney System (visual analogue scale: normal, 0; mild,1; moderate, 2; marked, 3) [25]. The mucosal thickness was measured at ten different arbitrarily selected points in the mid-antrum and mid-body. In addition, tissue sections were immunostained for H. pylori (rabbit polyclonal anti- H. pylori antibody, 1:20; DAKO, Kyoto, Japan), using an indirect immunoperoxidase method (peroxidase-labeled goat anti-mouse Ig antibody, 1:50, DAKO; peroxidase-labeled goat anti-rabbit Ig antibody, 1:50, DAKO).
The BrdU labeling was visualized using mouse monoclonal anti-BrdU antibody (1:50, DAKO), as described previously [4]. The labeling index was calculated by expressing the number of BrdU-positive epithelial cells as a percentage of the total number of epithelial cells from 10 different points, which were arbitrary selected, in the lesser curvature of the mid-antrum.
Statistical analyses
The statistical significance of the incidence of positive bacterial culture was evaluated using Fisher's exact test; the other factors were assessed by Student's t test after an analysis of variance. P values of less than 0.05 were considered to indicate significant differences. The results are expressed as the mean±standard deviation.
3. Results
Bacteriological and serological findings
In group A (H. pylori inoculation followed by JRF treatment), bacterial culture was positive in 5 of 20 gerbils (25 %) and then the density of H. pylori colonization was (3.8±2.3) X 102 CFU/stomach. Both of the incidence and density were significantly less than that in group B (H. pylori inoculation without JRF treatment. 84 % and (8.6±9.9) x 104 CFU/stomach, respectively). The titers of anti- H. pylori antibodies in group A was less than that in group B, however, the difference did not reach statistical significance at the time of sacrifice. (Table 1)
Summary of the experiment. HP: Inoculation of Helicobacter pylori. mono: mononuclear cells. F/T: the ratio of fundic mucosa to total glandular stomach length. *: statistically significant compared with group B (P<0.01).
| Bacterial culture | Antibod Y titers (AI) | Infiltration of | Mucosal thiskness | BrdU Labeling index | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| group | HP | Rice fluid | Incidence (%) | Density 100CFU | neutrophils | mono | Antrum (mm) | Corpus (mm) | F/T (%) | ||
| A | (+) | (+) | 25* | 3.8±2.3 | 50±60 | 2.3±0.5* | 1.2±0.4* | 0.35±0.1 | 1.2±0.3 | 43±6 | 4.0±0.2* |
| B | (+) | (-) | 84 | 860±990 | 78±70 | 2.9±0.3 | 2.5±0.5 | 0.39±0.1 | 1.2±0.1 | 38±15 | 6.4±0.7 |
| C | (-) | (+) | - | - | - | 0 | 0 | 0.19±0.01 | 0.61±0.1 | 81±4 | 0.9±0.2 |
| D | (-) | (-) | - | - | - | 0 | 0 | 0.20±0.02 | 0.62±0.1 | 82±5 | 0.9±0.1 |
Histopathological findings
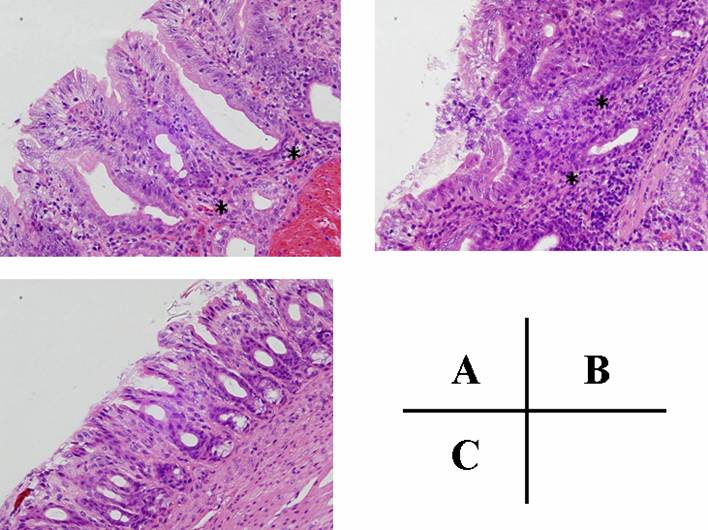
In animals from non- H. pylori inoculated groups (C and D), inflammatory cell infiltration into the lamina propria was negligible (Table 1 and Fig 2C). In contrast, active gastritis developed in the gastric mucosa of gerbils in group B (H. pylori inoculation only), which thus showed a marked mucosal infiltration of inflammatory cells (Table 1 and Fig 2B). In addition, superficial erosions in the antrum and irregularity of the pyloric glands were frequently observed in group B (Fig 2B). However, the degree of mucosal infiltration of inflammatory cells (both of neutrophilic polymorphonuclear and mononuclear cells) in group A (H. pylori inoculation followed by JRF treatment) was significantly less than that in group B (Table 1 and Fig 2A). In addition, no intestinal metaplasia or tumors in the gastric mucosa were observed in any groups based on the findings of this experiment.
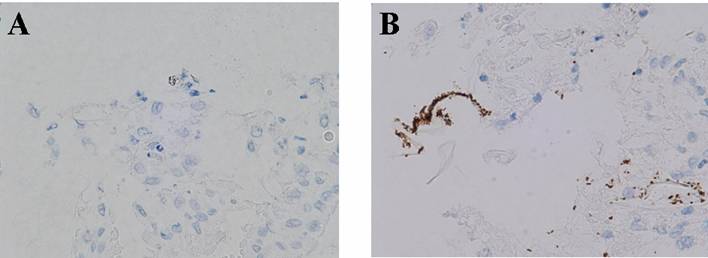
In addition to serological and bacteriological studies, the degree of infection of H. pylori was investigated by immunostainging. In group B, many H. pylori bacteria were detected on the surface of the mucosa (Fig 3B). On the other hand, only a small number of bacteria were found to exist in group A (Fig 3A).
The extent of epithelial proliferation was evaluated based on the BrdU labeling index. The BrdU labeling index in group A decreased significantly more than that in group B (Table 1), although it increased than in the non- H. pylori controls (groups C and D).
Histology of pyloric mucosa. H & E, X 40. (A) pyloric mucosa of Mongolian gerbil that was inoculated with H. pylori at 7 weeks of age and given rice-fluid from 2 weeks until 14 weeks after H. pylori inoculation (group A), (B) pyloric mucosa of Mongolian gerbil inoculated with H. pylori and given distilled water (group B), (C) normal (control) pyloric mucosa (group D). The degree of mucosal infiltration of inflammatory cells in group A (JRF treatment(+)) (asterisks) was less than that in group B (JRF treatment(-))(asterisks).
Immunostaining for H. pylori, X 80. (A) inoculation with H. pylori with treatment of rice-fluid, (B) inoculation with H. pylori without treatment of rice-fluid. Many H. pylori bacteria were detected on the surface of the mucosa in group B (JRF treatment(-)), while only a small number of bacteria were found to exist in group A (JRF treatment(+)).
4. Discussion
In the present study, we showed the suppressive effect of Japanese rice-fluid (JRF) on H. pylori infection in the Mongolian gerbil model. Both cultures and immunostaining of the stomach demonstrated a clear reduction in the H. pylori colonization. It is natural that the difference of anti- H. pylori antibody titers was not so large between the JRF -treated gerbils and the non-treated ones at the time of sacrifice, since several months are usually needed to show a significant decrease in the antibody titers after the eradication of H. pylori from the stomach [26]. In addition to the suppressive effect of H. pylori infection, JRF reduced mucosal inflammation and epithelial proliferation in the stomach of H. pylori-infected gerbils. It is likely that H. pylori-eradication caused a decrease in the degree of inflammation in the stomach, although there is a possibility that JRF itself has an anti-inflammatory effect on the gastric mucosa.
A positive association between a high rice intake and atrophic gastritis has been reported in some epidemiological studies [27]. However, the direct effect of rice on the promotion of atrophic gastritis is still unclear. There is a possibility that rice intake is only a marker of other dietary factors such as salt or ethnic/social factors. Therefore, it is not surprising that a substance produced from rice, namely JRF, showed both an anti-H.pylori and anti-inflammation effect on the stomachs infected with H. pylori. In addition, JRF also has completely different activity from rice itself: in our in vitro experiment, rice itself did not show any anti-H.pylori activity, while JRF showed a strong bactericidal activity against H. pylori [23].
Even though the triple therapy, using two antibacterial antibiotics and a proton pump inhibitor, is effective and its short duration helps maintain patient compliance, a considerable number of patients experience undesirable side-effects, such as diarrhea, epigastric pain, nausea and bloating [28]. Contrary to this, JRF is considered to demonstrate relatively few side-effects, since rice is eaten as a staple diet in most Eastern countries, including Japan. In addition, in the in vitro experiment, the antibacterial spectrum of JRF was found to be extremely narrow and it was only found to be active against some restricted organisms including H. pylori, however, it did not demonstrate any antibacterial activity against Escherichia coli, Klebsiella pneumoniae, K. oxytoca, or Enterococcus faecalis, which are all representative well known normal inhabitants of the human intestinal flora [23].
These safety characteristics of JRF may be therefore appropriate for the use in prevention of H. pylori infection. As chronic inflammation and increased cell proliferation are features common to the pathogenesis of many human cancers, and as these features seem to play a central role in the initiation and promotion of carcinogenesis, the suppression of inflammation and cell proliferation by JRF may thus be an effective modality for preventing H. pylori-induced carcinogenesis in the stomach.
In our previous study, a Rice Power Extract (RPE) showed a suppressive effect of gastric mucosal inflammation, but it did not show significant decrease in the viable H. pylori obtained from stomach samples [22]. In contrast, JRF showed a significant reduction of both H. pylori colonization and mucosal inflammation in the stomach. Although both of JRF and RPE are made from rice, their constituents could differe somewhat between them, since the manufacturing method is completely different: JRF is manufactured by incubation at high temperatures under high pressure and treatment with proteinase-amylase complex, while RPE is prepared by saccharization using Aspergillus oryzae and fermentation using Saccharomyces cerevisiae [22]. These manufacturing processes could therefore cause the differences observed in the anti-H. pylori activity between JRF and RPE. However, the mechanism of anti-H. pylori activity in JRF still remains to be elucidated. This mechanism of action should be clarified in future studies, and then the anti-H. pylori effect should thereafter be confirmed in human trials.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T. et al. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067-2069
2. Dixon MF. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol. 1991;6:125-130
3. Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulate for pyloric Campylobacter. Med J Aust. 1985;142:436-439
4. Maruta F, Ota H, Genta RM, Sugiyama A, Tatematsu M, Katsuyama T. et al. Role of N-Methyl-N-nitrosourea in the induction of intestinal metaplasia and gastric adenocarcinoma in Mongolian gerbils infected with Helicobacter pylori. Scand J Gastroenterol. 2001;36:283-290
5. Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642-648
6. Salih BA, Abasiyanik MF, Saribasak H, Huten O, Sander E. A follow-up study on the effect of Helicobacter pylori eradication on the severity of gastric histology. Dig Dis Sci. 2005;50:1517-1522
7. Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y. et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6:254-261
8. Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M. et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577
9. Shimizu N, Ikehara Y, Inada K, Nakanishi H, Tsukamoto T, Nozaki K. et al. Eradication diminishes enhancing effects of Helicobacter pylori infection on glandular stomach carcinogenesis in Mongolian gerbils. Cancer Res. 2000;60:1512-1514
10. Maruta F, Sugiyama A, Ishizone S, Miyagawa S, Ota H, Katsuyama T. Eradication of Helicobacter pylori decreases mucosal alterations linked to gastric carcinogenesis in Mongolian gerbils. J Gastroenterol. 2005;40:104-105
11. Axon ATR, Moayyedi P. Eradication of Helicobacter pylori: omeprazole in combination with antibiotics. Scand J Gastroenterol. 1996;31(Suppl215):82-89
12. Misiewicz JJ, Harris AW, Bardhan KD, Levi S, O'Morain C, Cooper BT. et al. One week triple therapy for Helicobacter pylori: a multicentre comparative study. Gut. 1997;41:735-739
13. Borody TJ, Shortis NP, Reyes E. Eradication therapies for Helicobacter pylori. J Gastroenterol. 1998;33(Suppl 10):53-56
14. Ling TK, Cheng AF, Sung JJ, Yiu PY, Chung SS. An increase in Helicobacter pylori strains resistance to metronidazole: a 5-year study. Helicobacter. 1996;1:57-61
15. Midolo PD, Lambert JR, Turnidge J. Metronodazole resistance: a predictor of failure of Helicobacter pylori eradication by triple therapy. J Gastroenterol Hepatol. 1996;11:290-292
16. Kamiji MM, de Oliveira RB. Mon-antibiotic therapies for Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2005;17:973-981
17. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564-582
18. Funatogawa K, Hayashi S, Shimomura H. et al. Antibacterial activity of hydrolyzable tannins derived from medicinal plant against Helicobacter pylori. Microbiol Immunol. 2004;48:251-261
19. Isogai E, Isogai H, Fujii B. et al. Inhibitory effect of Japanese green tea extracts on growth of canine oral bacteria. Bifidobac Microflora. 1992;11:53-59
20. Isogai H, Isogai E, Hyashi S. Antibacterial activities of catechin as phytomedical substance. Res Adv Antimicrob Agents Chhemother. 2000;1:13-18
21. Isogai E, Isogai H, Kimura K. et al. Effect of Japanese green tea extract on canine periodontal diseases. Microb Ecol Health Dis. 1995;8:57-61
22. Murakami M, Ota H, Sugiyama A, Ishizone S, Maruta F, Akita N. et al. Suppressive effect of rice extract on Helicobacter pylori infection in a Mongolian gerbil model. J Gastroenterol. 2005;40:459-466
23. Kawakami Y, Oana K, Hayama M, Ota H, Takeuchi M, Miyashita K. et al. In vitro bactericidal activities of Japanese rice-fluid against Helicobacter pylori strains. Int J Med Sci. 2006;3:112-116
24. Kumagai T, Yan J, Graham DY, Tozuka M, Okimura Y, Ikeno T. et al. Serum immunoglobulin G immune response to Helicobacter pylori antigens in Mongolain gerbils. J Clin Microbiol. 2001;39:1283-1288
25. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol. 1996;20:1161-1181
26. Kato S, Furuyama N, Ozawa K, Ohnuma K, Iinuma K. Long-term follow-up study of serum immunoglobulin G and immunoglobulin A antibodies after Helicobacter pylori eradication. Pediatrics. 1999;104:e22
27. Montani A, Sasazuki S, Inoue M, Higuchi K, Arakawa T, Tsugane S. Food/nutrient intake and risk of atrophic gastritis among the Helicobacter pylori -infected population of northeastern Japan. Cancer Sci. 2003;94:371-377
28. Myllyluoma E, Veijola L, Ahlroos T, Tynkkynen S, Kankuri E, Vapaatalo H. et al. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy - a placebo-controlled, double-blind randomized pilot study. Aliment Pharmacol Ther. 2005;21:1263-1272
Author contact
![]() Correspondence to: Dr. Satoshi Ishizone, Department of Surgery, Shinshu University School of Medicine, Asahi 3-1-1, Matsumoto, 390-8621, Japan. E-mail: ishizonemd.shinshu-u.ac.jp
Correspondence to: Dr. Satoshi Ishizone, Department of Surgery, Shinshu University School of Medicine, Asahi 3-1-1, Matsumoto, 390-8621, Japan. E-mail: ishizonemd.shinshu-u.ac.jp

 Global reach, higher impact
Global reach, higher impact