Impact Factor
ISSN: 1449-1907
Int J Med Sci 2006; 3(1):7-10. doi:10.7150/ijms.3.7 This issue Cite
Research Paper
Effect of antibodies on the expression of Plasmodium falciparum circumsporozoite protein gene
Centro de Malária e Outras Doenças Tropicais, UEI Malária, Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa, Rua da Junqueira, 96, 1349-008 Lisbon, Portugal
Received 2005-10-5; Accepted 2005-12-18; Published 2006-1-1
Abstract
Antibodies are known to play an important role in the control of malaria infection. However, they can modulate parasite development enhancing infection. The effect of anti-Plasmodium antibodies on the expression of circumsporozoite protein gene (csp) was investigated. Plasmodium falciparum 3D7 in vitro cultures were submitted to: i) anti- circumsporozoite protein monoclonal antibody (anti-CSP-mAb) [1μg/ml, 0.1μg/ml, 0.01μg/ml and 0.001μg/ml] and ii) purified IgG Fab fragment from a pool of malaria patients [1mg/ml and 1μg/ml]; and compared to control cultures. After 24h the number of ring infected erythrocytes was determined in order to calculate invasion efficacy. At 48h culture supernatant was collected, and the amount of circumsporozoite protein determined by ELISA, parasitaemia was calculated and cells were processed for RNA preparation. Expression of csp gene was quantified using Real time RT-PCR. There was an increase in parasite growth when treated with lower anti-CSP-mAb concentration, which was associated with lower csp expression, while 1μg/ml anti-CSP-mAb treatment presented a growth inhibitory effect accompanied by high csp expression.
Keywords: Plasmodium falciparum, circumsporozoite protein, erythrocyte invasion
1. INTRODUCTION
Recognition of pathogen molecules by antibodies leads to initiation of immune response. Binding of antibodies to pathogen molecules can disable pathogens or mediate destruction by other effector mechanisms. The role of antibodies during infection can vary from protection to pathogenesis. Further, antibodies can exert direct effect on the pathogen, modulating development and evasion to the immune system. Antibody modulation of expression and pathogen transcription levels has been described in virus. Antibodies to measles virus molecules expressed at the surface of infected cells can alter expression of polypeptides present in the cytoplasm of infected cells as well as those present at the plasma membrane [1]. Antibody modulation is not exclusive of virus, in vitro treatment of rabbit endothelial cells with specific anti-endothelial antibodies resulted in redistribution of antigens in the cell as well as co-redistribution of immunological unrelated antigens [2]. The inhibitory effect of antibodies on malaria development has been well documented. However, antibodies are also capable of modulating positively parasite development. Depending upon time of infection, anti-P. falciparum sporozoite antibodies can influence sporogonic development. When infected mosquitoes were membrane-fed, at day 5 post-infection (p.i.), with antibodies anti-P. falciparum sporozoite or anti- circumsporozoite protein (CSP) the absolute number of sporozoites recovered from the mosquito salivary glands at day 14 p.i. was significantly higher [3, 4]. Anti-gamete antibodies, which can suppress infectivity of P. vivax to the mosquito vector, at lower concentrations had the opposite effect, enhancing parasite numbers [5]. Nudelman and colleagues [6] also reported a similar effect of lower anti-Plasmodium antibody concentration. The number of mature liver schizonts increased up to 150% in the presence of mAbs anti-CSP of P. yoelii, which were suppressive at higher concentrations. Contradictory effects of antibodies on parasite development have also been described in the asexual stages of human malaria parasite P. falciparum. The presence of Kenyan immune serum in FC27 P. falciparum cultures had an inhibitory effect after 48h in culture. In contrast purified IgG, isolated from the same serum samples enhanced parasite growth up to 66% [7]. The circumsporozoite protein (CSP) is a major parasite surface protein during the sporogonic cycle. It is highly immunogenic, and in endemic areas high antibody titters against this protein are observed in circulating blood. This study sets to understand the role of anti-Plasmodium antibodies on the erythrocytic development of Plasmodium falciparum and their effect on the expression of csp gene.
2. Materials and Methods
Parasites and cultures
P. falciparum 3D7 was maintained in culture as described by Trager and Jensen [8]. Cultures were synchronised by two rounds of sedimentation using gelatine. Briefly, cultures of approximately 5% parasitised erythrocytes (PE) were centrifuged, the pellet obtained was resuspended in 9 volumes of pre-warmed gelatin solution (0.75% in Heppes-buffered RPMI 1640), and cultures were incubated for 45 min at 37 º C. The upper layer was collected and centrifuged; pellet was adjusted for a 5% haematocrite and cultured at 37ºC for 48h. A second round of synchronisation was performed prior to the assay.
Erythrocytes were sedimented by centrifugation at 2000g. Fresh washed non-infected erythrocytes were added to the pellet in order to obtain 2% PE. Culture haematocrite was adjusted to 5%. The obtained culture was divided in 3ml aliquots and centrifuged for 10min at 2000g. Supernatant was removed and replaced by fresh RPMI media supplemented with 10% heat inactivated serum and one of the following treatments: a) no other supplement - control; b) 1mg/ml and 1μg/ml purified IgG Fab fragment isolated from a pool of 750 malaria patients from Malawi, kindly provided by Prof. Hommel through Dr. Armada, Liverpool Scholl of Tropical Medicine, UK; c) 1 – 0.1 – 0.01 and 0.001 μg/ml anti-CSP monoclonal antibody (2A10-HA2) kindly provided by Dr. Wirtz, CDC, Atlanta, Georgia USA. Parasites were cultured for 24 or 48h in a CO2 rich environment. Blood smears were performed at 24 and 48 h. After 48h culture media and cells were collected for ELISA and RNA extraction respectively.
RNA preparation and cDNA synthesis
Extraction of total RNA was performed with Trizol (Life Technologies) according to the manufacturer's protocol. One microgram of total RNA from each sample was treated with 1U of DNase I (Life Technologies). Complementary DNA (cDNA) was synthesised in a 20 µl reaction with 1 µg treated RNA, 0.5μg oligo(dT)15, 50mM Tris-HCl pH 8.3, 75mM KCl, 1,5mM MgCl2, 10mM BSA, 0.5mM dNTP's, and 10U MMLV-RT (Life Technologies). RNA was retrotranscribed for 1 hour at 37ºC, denatured 5 min at 95ºC and quenched on ice. To certify the absence of genomic DNA (gDNA), duplicates of samples were incubated with a similar cDNA reaction mixture devoid of RT enzyme. No RNA controls were included for each reaction mixture used.
Real-Time Reverse Transcriptase-PCR Analysis
Gene-specific primers for P. falciparum ldh [9] and csp [10] genes were synthesised by Life Technologies and used in a reaction mixture using SYBR® Green PCR Core reagents kit (PE Applied Biosystems). PCR reactions were performed in a volume of 20µl containing the following primer concentrations: ldh (0.3mM -fwd, 0.05mM-rev), csp (0.1mM -fwd, 0.3mM-rev). Each reaction contained 1µl of cDNA template. Amplification and detection of specific products was performed with the GeneAmp® 5700 system (PE Applied Biosystems) using the following cycle profile: 1 cycle at 48°C for 30 min, 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 s, and 60°C for 1 min.
Quantification relies on the comparison of the critical threshold cycle (Ct) of an unknown sample against a standard curve of known quantities. Ct is the amplification cycle at which the fluorescence becomes detectable and is inversely proportional to the logarithm of the initial amount of template DNA. For each reaction a standard curve was plotted with Ct values obtained from amplification of known quantities of gDNA (100, 10, 1, 0.1 and 0.01ng) isolated from P. falciparum 3D7 clone. Concentration of DNA was determined by spectrophotometric analysis of optical density at 260nm (GeneQuant, Pharmacia) and standard DNA solutions were prepared. A standard curve was used to transform Ct values to the relative number of DNA molecules. Triplicates of each sample and standard curve were performed in all assays. The quantity of cDNA for csp was normalised to the quantity of the house keeping gene ldh cDNA in each sample. Melting curves were used to determine the specificity of PCR products.
ELISA for detection of circumsporozoite protein (CSP)
Microtitration plates were coated with PBS containing 0.2μg/well capture antibody (Pf-CAP, lot. TF097, CDC, Atlanta) and incubated overnight at room temperature. Coating solution was replaced with blocking buffer (0.5% Casein, 0.1N NaOH, PBS pH7.4, 20mg/l phenol red) and incubated at RT for one hour. P. falciparum 3D7 48h culture supernatant was centrifuged at 2000g to remove debris. Blocking buffer in the wells was replaced with 100μl of culture supernatant and incubated for 1h. Fresh culture medium was used as negative controls. For each plate a titration (100 – 0.75pg) of recombinant positive control (Pf-PC, lot R32, CDC, Atlanta) was added. The wells were then washed twice with PBS Tween20. Peroxidase conjugate antibody diluted in blocking buffer (0.05μg) was added to each well. After 2h of incubation, plates were washed 3 times with PBS Tween20, and peroxidase substrate added. The absorbance was read at 414 nm. The standard curve was used to quantify the amount of CSP present in the samples. ELISA mAbs and positive control were acquired from Dr. Wirtz, CDC, Atlanta, GA USA.
Statistical analyses
Data analysis was performed using SPSS statistical program. The Wilcoxon signed-rank test, for 2 matched samples was used to evaluate differences between groups. The significant level was taken as p<0.05.
3. Results
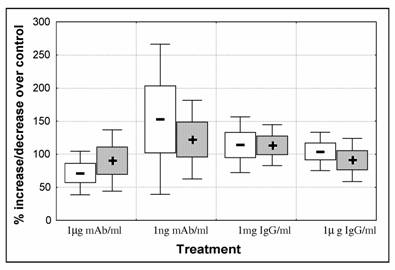
At starting of experiments parasitaemia was set to approximately 2% PE. Blood smears were performed to confirm parasitaemia that varied from 1.9 to 2.1 (percentage of ring stages/ early trophozoite varied 87 to 97%). After 48h of culture all controls showed an increase in parasite number with total parasitaemia varying from 2.9 to 4.1. There were no statistical significant differences between control and treated parasites. However, we observed a decrease in the mean number of parasites when treated with 1μg mAb/ml. Anti-CSP-mAb had contradictory effect depending on concentration and although mAb at lower concentration seems to have a stimulatory effect, in this group the variability between experiments was high. The opposite was observed in parasites treated with IgG (Fig. 1).
P. falciparum 3D7 in vitro growth. Parasites were submitted for 48h to different antibody treatments: 1μg/ml and 0.001μg/ml anti-CSP monoclonal antibody (mAb); 1mg/ml and 1μg/ml purified IgG Fab fragment isolated from a pool of 750 malaria patients from Malawi. Cultures were set for 2% parasitaemia (87-97 % ring stage parasites) and 5% heamatocrit and were cultured for 48 hours. Percentage of parasite increase/decrease was calculated by dividing parasitaemia of treated cultures over control cultures (control = 100%). Parasitaemia of 3 replicates per experiment was counted. Data represents mean increase of ring stage parasites ( - ) and schizonts (+) of 5 independent experiments. Boxes represent standard error of the mean [open – ring stages; solid – schizonts] and whiskers represent standard deviation of the mean
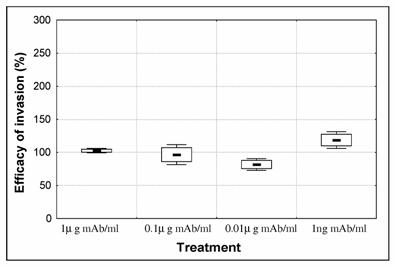
In order to investigate if antibodies were exerting an effect on invasion, serial dilutions of mAb anti-CSP were tested in the culture medium. After synchronisation, the percentage of schyzonts varied from 88 to 90% [Initial % parasitaemia was 1.98 +/- 0.11 (mean +/- SD)]. Parasites were cultured for 24h and ring-stage infected erythrocytes were counted [control ring-stage % parasitaemia was 3.07 +/- 0.73 (mean +/- SD)]. Efficacy of invasion was calculated as the percentage of ring infected erythrocytes of test cultures over the percentage of ring infected erythrocytes in control culture (Fig. 2). When anti-CSP mAb was present in the culture we observed a dose dependent decrease in invasion, that was inverted when parasites were treated with the lowest concentration (1ng/ml mAb) presenting an increase of invasion. Although, close to significant level this increase was not significantly different from the control (p=0.0578).
Effect of anti-CSP monoclonal antibody (mAb) on P. falciparum 3D7 merozoite invasion. Purified schizonts (88-90 %) were cultured for 24h with human erythrocytes in order to measure the ability of merozoite to invade erythrocytes in the presence of 1- 0.1 – 0.01 and 0.001μg/ml anti-CSP monoclonal antibody (mAb). The number of ring stage infected erythrocytes of 3 replicates was counted. Efficacy of invasion was calculated as the percentage of ring infected erythrocytes of treated cultures over the percentage of ring infected erythrocytes in control culture (control = 100%). Boxes represent standard error of the mean and whiskers represent standard deviation of the mean
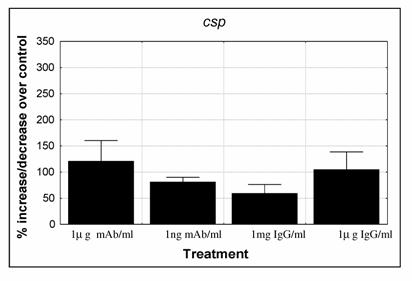
No amplification was observed when cDNA controls, that lack the reverse transcriptase enzyme, were used in the real time PCR reactions, demonstrating that there was no gDNA contamination. PCR reaction triplicates showed no major differences among them and expression levels of the internal control were similar in the different experiments. The expression levels of csp gene were depressed when parasites were submitted to 1ng/ml mAb (p=0.042) and 1mg/ml IgG Fab fragment (p=0.0796), while with the other treatments expression of csp was maintained similar or higher than the control (Fig. 3). Although differences between experiments were observed, a similar trend was seen between them.
Expression levels of P. falciparum 3D7 gene that codes for the circumsporozoite protein (csp). Parasites were cultured with: 1μg/ml and 0.001μg/ml anti-CSP monoclonal antibody (mAb); 1mg/ml and 1μg/ml purified IgG Fab fragment isolated from a pool of 750 malaria patients from Malawi. Cultures were set for 2% parasitaemia (87 to 97% ring stage parasites) and 5% haematocrit and were cultured for 48 hours. Quantification of cDNA was determined against a standard curve and normalised by the amount of ldh expression. Percentage of increase/decrease expression was calculated by dividing treated cultures over control cultures (control = 100%). Values represent the mean and standard error of 4 independent experiments
The presence of CSP was quantified in culture supernatants using a sandwich ELISA. Levels of CSP production into the media were very low in 48h culture supernatant [varying from 0.85 pg/ml +/- 0.02 to 0.93 pg/ml +/-0.01 (mean +/- SD)] and showed no differences between treatments.
4. Discussion
Antibodies are known to play an important role in the control of malaria infection. Since the early studies demonstrating that antibodies transferred from immune individuals diminish P. falciparum parasitaemia [11] a lot of effort has been put forward to identify parasite epitopes and mechanisms of action of antibody-mediated immune response to malaria. Besides their role in infection control, antibodies can modulate parasite development in the sporogonic [3 - 5], exoerythrocytic [6] and erythrocytic [7] cycle. However, there is almost no information on the effect of antibodies on the expression of Plasmodium immunogenic molecules.
There are evidences that antibodies can interfere with parasite multiplication. An increase on sporozoite number recovered from the salivary glands when mosquitoes were fed on anti-Plasmodium antibodies was observed [3, 4] and IgG isolated from Kenyan immune adults enhanced parasite growth in culture while the serum from which they were isolated had an inhibitory effect [7]. The number of mature liver schizonts increased in the presence of mAbs anti-CSP of P. yoelii and the authors suggested that enhancement of infection could be a consequence of an interaction between the parasite and the host cell membrane [6]. Surface changes were also proposed by Peiris et al. [5] to explain enhanced transmission of P. vivax to the mosquito vector. In our model interactions at surface level are unlikely as CSP is not observed at the surface of blood stage parasites [12].
CSP is a major protein at the sporozoite stage of Plasmodium and is normally referred as stage specific. However, the presence of Plasmodium csp transcripts have been described in erythrocytic forms [13, 14] and Cochrane et al. [12] isolated a protein from Plasmodium berghei erythrocytic stages that reacted with anti-CSP-mAb and had similar molecular mass and isoelectric points as the CSP isolated from the sporozoite stage. Although from literature we know that csp is not an essential gene during P. berghei blood stage development, as disruption of csp gene had no effect on blood stage infection [15], our data suggests that CSP might play a role during the erythrocytic development of the parasite.
Several functions have been described for CSP, sporozoite gliding motility [16] recognition and binding to the salivary glands [17] and more recently Thathy et al. [18] have demonstrated that CSP is essential for sporozoite morphogenesis in the oocyst. The effect observed at the present work indicates that availability of CSP during erythrocytic stages can interfere with parasite multiplication and invasion, suggesting that CSP is likely to be involved indirectly on erythrocyte invasion. The immune localisation of the CS-like protein described by Cochrane et al. [12] only found at the micronemes of merozoites of mature invasive blood stages is also indicative that CSP might play a role in the early stages of parasite interaction with the host red blood cell.
Antibody dependent enhancement of viral replications has been described in several viruses (reviewed by Sullivan [19]). This phenomenon has been associated, with the ability of viruses to interfere with the activity of antiviral pathways, cytomegalovirus inhibited class II major histocompatibility complex expression in endothelial cells and fibroblasts [20] and Ross River virus interfered with transcription of key antiviral genes through targeting the transcription factors IRF-1 and NF-κB [21]. As virus relies on host cell machinery for multiplication, anti-viral antibodies exert their effect on host cell. In malaria, the presence of CSP in the cytoplasm of HepG2 cells and macrophages has been described to inhibit protein synthesis in the target cells, through interaction with ribosomes [22]. Based on their observations the authors suggested that malaria sporozoites are able to control the protein synthesis machinery in cells of the vertebrate host, which could explain the differences in the levels of expression observed in our study.
The effect of antibodies directed to an antigen predominantly expressed in a different life cycle stage, could contribute to parasite escape from destruction by influencing the efficacy of mechanisms such as host cell invasion. Parasite manipulation of their expression machinery by host immune system molecules might be a parasite mechanism that evolved to avoid potential lethal effects of neutralising Ab.
Acknowledgements
We are grateful to Claudia Melo for performing the CSP – ELISAs. We would like to thank Prof. Hommel and Dr. Armada for providing purified IgG Fab fragment. CSP detection ELISA was provided by Dr. Wirtz, CDC, Atlanta, GA, USA. We would also like to thank Encarnação Horta for technical assistance.
This work was supported by research funds from the project POCTI/35815/MGI/2000.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Fujinami RS, Oldstone MB. Alterations in expression of measles virus polypeptides by antibody: molecular events in antibody-induced antigenic modulation. J Immunol. 1980 ;125:78-85
2. Yuzawa Y, Brentjens J R, Brett J. et al. Antibody-mediated redistribution and shedding of endothelial antigens in the rabbit. J Immunol. 1993 ;150:5633-5646
3. Vaughan JA, Do Rosario V, Leland P. et al. Plasmodium falciparum; ingested anti-sporozoite antibodies affect sporogony in Anopheles stephensi mosquitoes. Exp Parasitol. 1988 ;66:171-182
4. Hollingdale MR, do Rosario V. Malaria transmission-enhancing activity in mosquitoes by mammalian host anti-sporozoite antibodies. Exp Parasitol. 1989 ;68:365-368
5. Peiris JS, Premawansa S, Ranawaka MB. et al. Monoclonal and polyclonal antibodies both block and enhance transmission of human P vivax malaria. Am J Trop Med Hyg. 1988 ;39:26-32
6. Nudelman S, Renia L, Charoenvit Y. et al. Dual action of anti-sporozoite antibodies in vitro. J Immunol. 1989 ;143:996-1000
7. Shi YP, Udhayakumar V, Oloo AJ. et al. Differential effect and interaction of monocytes, hyperimmune sera, and immunoglobulin G on the growth of asexual stage Plasmodium falciparum parasites. Am J Trop Med Hyg. 1999 ;60:135-141
8. Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976 ;193:673-675
9. Moormann AM, Hossler PA, Meshnick SR. Deferoxamine effects on Plasmodium falciparum gene expression. Mol Biochem Parasitol. 1999 ;98:279-283
10. Alloueche A, Silveira H, Conway DJ. et al. High-throughput sequence typing of T-cell epitope polymorphisms in Plasmodium falciparum circumsporozoite protein. Mol Biochem Parasitol. 2000 ;106:273-282
11. Cohen S, McGregor IA, Carrington SC. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 ;192:733-777
12. Cochrane AH, Uni S, Maracic M. et al. A circumsporozoite-like protein is present in micronemes of mature blood stages of malaria parasites. Exp Parasitol. 1989 ;69:351-356
13. Levitt A, Dimayuga FO, Ruvolo VR. Analysis of malarial transcripts using cDNA-directed polymerase chain reaction. J Parasitology. 1993 ;79:653-662
14. Ruvolo V, Altszuler R, Levitt A. The transcript encoding the circumsporozoite antigen of Plasmodium berghei utilizes heterogeneous polyadenylation sites. Mol Biochem Parasitol. 1993 ;57:137-150
15. Menard R, Sultan AA, Cortes C. et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997 ;385:336-340
16. Stewart MJ, Vanderberg JP. Malaria sporozoites release circumsporozoite protein from their apical end and translocate it along their surface. J Protozool. 1991 ;38:411-421
17. Sidjanski SP, Vanderberg JP, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol. 1997 ;90:33-41
18. Thathy V, Fujioka H, Gantt S. et al. Levels of circumsporozoite protein in the Plasmodium oocyst determine sporozoite morphology. EMBO J. 2000 ;21:1586-1596
19. Sullivan NJ. Antibody-mediated enhancement of viral disease. Curr Top Microbiol Immunol. 2001 ;260:145-169
20. Miller DM, Rahill BM, Boss JM. et al. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J Exp Med. 1998 ;187:675-683
21. Lidbury BA, Mahalingam S. Specific ablation of antiviral gene expression in macrophages by antibody-dependent enhancement of Ross River virus infection. J Virol. 2000 ;74:8376-8381
22. Frevert U, Galinski MR, Hugel FU. et al. Malaria circumsporozoite protein inhibits protein synthesis in mammalian cells. EMBO Journal. 1998 ;17:3816-3826
Author contact
![]() Corresponding address:
Corresponding address:
Henrique Silveira, e-mail: hsilveiraunl.pt. Tel: +351 21 3652657 Fax: +351 21 3622458

 Global reach, higher impact
Global reach, higher impact