Impact Factor
ISSN: 1449-1907
Int J Med Sci 2005; 2(1):36-40. doi:10.7150/ijms.2.36 This issue Cite
Review
Natural History and Clinical Consequences of Hepatitis B Virus Infection
1 Division of Gastroenterology and Hepatology, Elmhurst Hospital Center of Mount Sinai Services, Mount Sinai School of Medicine, New York, USA
2 Division of Gastroenterology, Mount Sinai Hospital, Mount Sinai School of Medicine, New York, USA
Received 2004-10-1; Accepted 2005-1-2; Published 2005-1-5
Abstract
Despite the existence of Hepatitis B vaccination, hepatitis B virus (HBV) infection is still prevalent worldwide and accounts for significant morbidity and mortality. It is encouraging that majority of patients do recover from the acute infection, however, those that progress to chronic disease state is at great risk of developing complications such as hepatocellular carcinoma, cirrhosis and liver failure. Hepatitis B virus infection can be influenced by many factors such as host immune status, age at infection, and level of viral replication. The discovery about the existence of various genotypes and its association with different geographic distribution as well as the knowledge regarding mutant species has aid us in better understanding the nature of HBV infection and in delivering better care for patients. It is especially important to recognize those individuals with HBeAg-negative chronic HBV as they have a poorer prognosis compare with their counterparts, HBeAg-positive. Tremendous progress has been made over the years in understanding the behavior and clinical course of the disease; however, the natural history of HBV is complex and we still have much to explore and learn.
Keywords: Hepatitis B, natural history, cirrhosis, hepatocellular carcinoma
1. Background
Despite the presence of hepatitis B vaccine, new HBV infection remains common. Primary HBV infection in susceptible individuals can be either symptomatic or asymptomatic, the latter being often the case, especially in young individuals; but rarely, fulminant hepatitis can develop during acute phase. Most primary infections are self-limited with clearance of virus and development of immunity. However, an estimated 3% to 5% of adults and up to 95% of children develop chronic HBV infection. Persistent infection can also be either symptomatic or asymptomatic; those with elevated liver chemistries and abnormal biopsies are termed as having chronic hepatitis B and those with normal studies are labelled as chronic HBV carriers. Long-term infection increases risk of developing cirrhosis and HCC.
2. HBV Genotypes and Mutants
DNA sequence of HBV isolates has shown the existence of 8 viral genotypes A-H and these varies in geographic distribution (Table 1). Genotype A is found primarily in North America, Northern Europe, India, and Africa. Genotype B and C are common in Asia; genotype D, in southern Europe, the Middle East, and India; genotype E, in West Africa and South Africa; genotype F, in South and Central America; genotype G, in the United States and Europe [1]. Genotype H was recently identified in individuals from Central America and California [2]. Several genotypes may be associated with the severity of the disease but the relationship between the genotype and the developing hepatocellular carcinoma has not been established. In China and Japan, some studies have found more severe liver disease to be associated with genotype C than compare with genotype B [3], other studies have found no such association [4,5]. There is some evidence that shows HBeAg seroconversion occurs at a younger age among individuals infected with genotype B [3, 5, 6]. Genotype D has been associated with anti-HBe-positive chronic hepatitis B infection in the Mediterranean region [7].
Genotypes of HBV and Geographic Distribution
| Genotype | Geographic Distribution |
| A | Africa, India, Northern Europe, United States |
| B | Asia, United States |
| C | Asia, United States |
| D | India, Middle East, Southern Europe, United States |
| E | West and South Africa |
| F | Central and South America |
| G | Europe, United States |
| H | Central and South America, California in United States |
HBV has a reported mutation rate of 10 times greater compare with other DNA viruses. These mutations can occur naturally as well as due to selective pressure from antiviral therapy. There are five clinically relevant HBV types: wild-type HBV, precore mutants, core promoter mutants, tyrosine-methionine-aspartate-aspartate (YMDD) mutants induced by lamivudine treatment, and asparagine to threonine (rtN236T) mutants recently identified in patients with adefovir treatment. In a study carried out in the United States, the precore variant of HBV was rarely found in association with genotype A, but it was found in almost 50% of those with genotype C and in >70% of individuals with genotype D. Those with precore variant and core promoter mutations had higher HBV DNA levels in sera than those persons without these mutations. It is observed that flares in chronic HBV have been associated with increases in concentrations of precore mutation in proportion to wild-type HBV. Exacerbations have been thought to subside with time when the genetic heterogeneity disappears and patients become exclusively infected with precore HBV [8]. During the treatment of chronic hepatitis B by lamivudine, drug resistance may develop, which is mediated by point mutations with the YMDD motif at the catalytic center of the viral reverse transcriptase. With increase in the mutant viral load, patients can sustain further liver injury. The YMDD mutant level will decrease after stopping lamivudine. Viral mutation also occurs in patients on adefovir treatment during their second year therapy at the rate of 2.5%. The mutation has been reported as asparagine to threonine mutation (rtN236T), downstream of the YMDD motif. It is not clear yet if rtN236T mutant can induce further liver damage.
3. HBV Transmission and Vaccination
Perinatal or horizontal infection early in childhood are the main routes of HBV transmission in high endemic region, such as Asia, Africa, Pacific Islands and the Arctic and the rate of HBsAg positivity ranges from 8% to 15%. In the low endemic area, such as Western countries, HBV is predominantly a disease of adolescents and adults as a result of high risk sexual behavior or injection drug use and the rate of HBsAg positivity is less than 2% [9]. HBV infection frequently is waxing and waning, it goes through alternating cycles of replicative and non-replicative phases.
Since the availability of hepatitis B vaccination in 1982, it has had some dramatic impact on the outcome of liver disease. For example, in Alaska, the proportion of HBV carriers who were HBsAg positive in the population declined from 35% to 3% in 15 years after the introduction of the vaccination program in newborns and the completion of a mass vaccination catch up program [10]. In Taiwan, the universal hepatitis B vaccination in newborns has both dramatically decreased prevalence of HBsAg as well as incidence of HCC in this age group.
4. Stages of HBV Infection
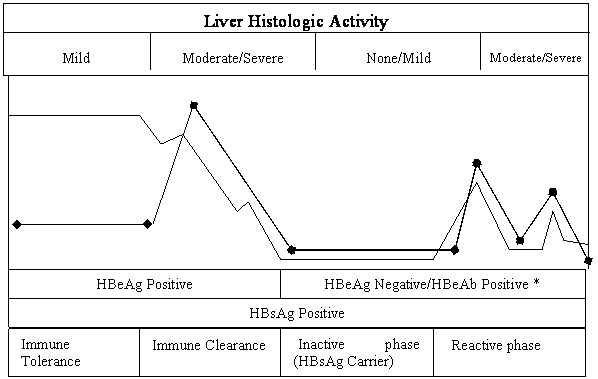
An individual can develop hepatitis B infection that is acute and achieve complete immune clearance of virus yielding lifelong immunity; however, an alternate fate of the host is the development of chronic hepatitis B. There are three stages of HBV infection based on viral-host interaction, namely, the immune tolerant phase, the immune clearance phase, and the inactive carrier phase with or without reactivation (Fig 1.). After acute infection of HBV, some patients may remain HBeAg positive with high levels of serum HBV DNA, little or no symptoms, normal ALT levels and minimal histological activity in the liver, this phenomenon is known as the immune tolerance phase. This phase is typical of infection in children and young adults. It usually lasts for 2-4 weeks, but can last for years in those who acquired the infection during the perinatal period [11]. Individuals in this group are highly contagious and can transmit HBV easily. When the tolerogenic effect is lost during the immune tolerant phase, immune-mediated lysis of infected hepatocytes become active and patients enter the second stage defined as immune clearance phase, the HBV DNA level decreases and ALT level increases. The duration of clearance phase lasts from months to years. This is followed by the carrier stage, in which seroconversion of HBeAg to HBeAb occurs, HBV DNA becomes non-detectable or at low level and ALT is usually normal, reflecting very low or no replication of HBV and mild or no hepatic injury. The inactive carrier stage may last for years or even lifetime. Patients in this stage can have spontaneous resolution of hepatitis B and develop HBsAb, but a portion of them may undergo spontaneous or immunosuppression-induced reactivation of chronic hepatitis, featuring elevated ALT, high level of DNA, moderate to severe liver histological activity, and with or without HBeAg seroreversion.
5. Clinical Spectrum of HBV Infection
Primary Infection –Subclinical Infection and Acute hepatitis B
Majority of HBV infection in children are subclinical versus those in adults, about 30% to 50% develop acute icteric hepatitis. Those who recover should acquire lifelong immunity. However, a subset of patients will be chronically infected and very few patients (0.1% - 0.5%) can develop fulminant hepatitis. In primary infection, HBsAg becomes detectable after 4 to 10 weeks of incubation period, followed by antibodies against the core antigen (HBcAb) in IgM form during early period [2]. Viremia is well established by the time HBsAg is detected (usually from 10^9 to 10^10 per milliliter) [12]. Circulating HBeAg becomes detectable in most cases. When liver injury occurs in acute infection, alanine aminotransferase levels do not increase until after viral infection is well established, reflecting the time required to generate the T-cell-mediated immune response that triggers liver injury. Once this response has commenced, viral titer in blood and liver begins to drop. With clearance of the infection, HBsAg and HBeAg disappear, and free HBsAb become detectable. Presence of HBsAg, HBeAg, and high DNA level for greater than 6 months implies progression to chronic infection. When persistent infection establishes, the serology markers like HBeAg, HBeAb and HBsAb can be positive or negative except HBsAg and HBcAb (IgG form) remain positive.
HBeAg positive chronic hepatitis B
Age at the time of infection is a strong determinant of chronicity, the earlier the acquisition of infection, the higher probability of developing chronic infection. Levels of viremia in chronic infection are generally significantly lower than during primary infection. In adult acquired disease, the early phase of infection often is accompanied by significant disease activity with elevated ALT levels versus those who acquired the infection perinatally usually have normal ALT levels. Many patients with perinatal infection enter the immunoactive phase and develop HBeAg positive chronic hepatitis with elevated ALT levels only after 10-30 years of infection [13]. A key event in natural history of progression is HBeAg seroconversion to HBeAb with marked reduction of HBV replication followed by gradual histological improvement [14]. In studies, the observed clearance of HBeAg is about 50% and 70% within 5 and 10 years of diagnosis respectively [9]. Most studies found the mean annual rate of spontaneous seroconversion is 8% to 15% in individuals with active liver disease, but those with normal ALT levels tend to have smaller annual conversion rate of 2% to 5%.
HBeAg negative chronic hepatitis B
A proportion of patients who undergo HBeAg seroconversion demonstrate a recurrence of high HBV DNA levels and intermittent or persistent ALT level elevations. These individuals have a naturally occurring mutant form of HBV that does not produce HBeAg, due to a mutation in the precore or core promoter region. Most frequent precore mutation is a G-A change at nucleotide 1896 (G1896A) which creates a stop codon and results in loss of HBeAg synthesis; the most common core promoter mutation involves a 2 nucleotide substitution at nucleotide 1762 and 1764 [15]. HBeAg-negative carriers are a heterogeneous group and most of them have low viral DNA levels, relatively normal levels of alanine aminotransferase, and a fair prognosis. However, in Asia, Middle East, Mediterranean basin and southern Europe, about 15% to 20% of these carriers have elevated alanine aminotransferase and viral DNA [16]. HBeAg-negative chronic hepatitis B (precore mutant) emerges as the predominant species during the course of typical HBV infection with wild-type virus and is selected during the immune clearance phase (HBeAg seroconversion) [17]. The development of HBeAg-negative chronic hepatitis B can occur either close to HBeAg seroconversion or decades later. There are 2 main patterns of disease activity in this subgroup of patients: 30%-40% of patients experience persistent elevated ALT levels (3-4 folds) and the remaining 60%-70% patients can have erratic ALT patterns with frequent flares. Sustained spontaneous remission is rare (6% to 15%) in these individuals, and spontaneous HBsAg clearance is only about 0.5% per year [18]; hence, long-term prognosis is poorer among HBeAg-negative individuals than compare with their counterparts who are HBeAg-positive.
Inactive HBsAg carrier state
Inactive HBsAg carrier state is diagnosed by HBeAg negativity with anti-HBe positivity, undetectable or low HBV DNA level, repeatedly normal ALT, and normal or minimal histological evidence of damage [19]. The prognosis of the inactive carrier is generally good and well supported by long-term follow-up studies [20, 21, 22]. An estimated 20% to 30% of HBsAg carriers may develop reactivation of hepatitis B with elevation of biochemical levels, high serum DNA level with or without sero-reversion to HBeAg. Recurrent episodes of reactivation or sustained reactivation can occur and contribute to progressive liver disease and decompensation. Frequently, HBV reactivation is usually asymptomatic, but it may mimic acute viral hepatitis. On the other hand, some carriers eventually become HBsAg negative and develop HBsAb. It is reported that the incidence of delayed HBsAg clearance is close to 1% to 2% per year in Western countries, but only 0.05% to 0.8% per year in endemic areas where infection was often acquired during childhood. Apparently, women and older carriers have higher clearance of HBsAg. Reactivations of HBV replication in patients who receive immunosuppressive therapy or cytotoxic chemotherapy have been reported in the range of 20% to 50% in chronic carriers [23, 24]; from experience, the combined use of corticosteroid in chemotherapies significantly increases the risk of reactivation [23]. However, these flares in the immunosuppressed individuals rarely result in significant hepatic decompensation.
6. Long-term Sequelae of Chronic Hepatitis B
Cirrhosis
Following the diagnosis of chronic hepatitis B, the survivals in these patients are estimated to be 100% at 5 years. However, cirrhosis and hepatoma are two major long-term complications of chronic HBV infection that significantly increases morbidity and mortality. In patients without cirrhosis, if untreated, the incidence of liver related death is low and ranges from 0 to 1.06 per 100 person years. The mortality rate at 5 years is 16% for those with compensated cirrhosis and is 65% to 86% for decompensated cirrhosis [25, 26]. In untreated individuals with predominantly HBeAg positive chronic hepatitis B, the incidence of cirrhosis ranges from 2 to 5.4 per 100 person years with a 5-year cumulative incidence of cirrhosis of 8% to 20% [9]. A higher rate of cirrhosis has been reported in HBeAg-negative as compared to HBeAg-positive patients. Also, older age and persistent viral replication are predictors for development of cirrhosis as well as mortality. Genotype C is associated with a higher risk of cirrhosis than genotype B based on studies in Asian patients [27]. The presence of any other independent hepatotoxic factors such as alcohol ingestion, HCV co-infection can contribute to progression to cirrhosis. Once cirrhosis is established, individuals can decompensate over time. In the EUROHEP cohort study, the 5-year cumulative incidence of hepatic decompensation was 16%, the incidence per 100 person years was 3.3 and the mean interval between the time of diagnosis of cirrhosis and the onset of first episode of decompensation was 31 months (range 6-109) [28]. After decompensation, the survival drops to 55% to 70% at 1 year and to 14% to 28% at 5 years; Interestingly, an improvement in liver function activity has been observed in those individuals who subsequently lose their HBsAg positivity.
Hepatocellular Carcinoma
The development of hepatocellular carcinoma (HCC) and liver failure are main cause of death from chronic hepatitis B. Various factors involving the host and the virus may contribute to the development of hepatocellular carcinoma (Table 2). It is estimated that over 500,000 people die each year from the consequence of HBV infection [9]. HCC incidence is three to six times higher in males than in females, suggesting a tumorigenic effect of androgens [10, 29]. Several studies have indicated that older age (>45 years) is an important determinant of HCC, this may either reflect a longer duration of viral infection and liver disease or age may be an independent risk factor. Having a first degree relative with HCC, the presence of cirrhosis, and reversion activity are all thought to contribute to HCC development [10, 29, 30, 31]. Chronically infected subjects have a 100 times increased risk of hepatocellular carcinoma compare with non-carriers [20]. A recent study suggested positive HBsAg increased one's risk of developing HCC by 10 folds, and with positive HBeAg, HCC is significantly increased by 60 folds. Moreover, a detectable HBV DNA level yields a 4 fold increase risk of HCC [32]. The additional use of alcohol, consumption of aflatoxin in diet and co-infection with HCV or HDV are independent factors for HCC in HBV infected patients. Unlike hepatitis C, development of HCC in hepatitis B patients does not require preceding cirrhosis. Hence, it is advocated that all HBV infected patients, regardless of cirrhosis status, should get screening for HCC every 6 months with alpha-fetoprotein (AFP) and liver sonogram. Currently, there is no consensus as to when such screening should commence, but it is reasonable to start screening immediately once these patients seek medical attention.
Independent Risk Factors for the Development of HCC in HBV infection
| Types | Risk Factors |
| Host | Male |
| Older age (>45 years old) | |
| First degree relatives with HCC | |
| Clinical | HBeAg positive |
| Detectable HBV DNA | |
| Cirrhosis | |
| Persistent HBV infection (HBsAg positive) | |
| Viral and environmental | Coinfection with HCV or HDV |
| Alcohol intake | |
| Aflatoxin in Diet |
7. Conclusion
It has been encouraging to witness the recent discoveries in HBV infection with insights into the existence of genotype subgroups, mutant variants, knowledge regarding host, viral and environmental factors on the disease course, as well as advances in new treatment modalities. However, despite the much progress in understanding the natural history of HBV infection, we still have a long way to go before we can conquer hepatitis B infection. For instance, more studies are needed to clarify whether there is an association between genotype, mutant variants and the development of hepatocellular carcinoma. In the HBeAg-positive subgroup, there still lacks a consensus on how to manage these patients when they present with signs of mild liver disease activity with alanine aminotransferase less than 2 fold increase; future studies with longer follow-up may help us gain knowledge about the HBV behavior in these individuals. There is much more to be understood about mutations and their impacts on the clinical course and long-term outcome of hepatitis B infection. For instance, it has been suggested that mutations can arise from vaccine-induced antibodies and this renders the immune response generated by the vaccination ineffective. Therefore, mutations may play a key role in the difficulties of managing hepatitis B infection. Hence, further research and understanding in this sector may bring exciting new information and better understanding of the natural history of HBV and supplement our existing armamentarium to combat this persistent worldwide prevalent disease.
Conflict of interest
Dr. Pan is on the panel of speaker's bureau for Novartis Pharmaceuticals USA and received research grand support from Schering-Plough Corporation. Dr. Zhang has no disclosable financial arrangements or interest with any corporations.
References
1. Kidd-Ljunggren K, Miyakawa Y. et al. Genetic variability in hepatitis B viruses. J Gen Virol. 2002 ;83:1267-1280
2. Arauz-Ruiz P, Norder H, Robertson BH. et al. Genotype H: A new meridian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002 ;83:2059-2073
3. Kao JH, Chen PJ, Lai MY, Chen DS. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. J Clin Microbiol. 2002 ;40:1207-1209
4. McMahon BJ, Holck P, Bulkow L. et al. Serologic and clinical outcomes of 1536 Alaska natives chronically infected with hepatitis B virus. Ann Internal Med. 2001 ;135:759-768
5. Sumi H, Yokosuka O, Seki N. et al. Influence of hepatitis B virus genotypes on the progression of chronic hepatitis B liver disease. Hepatology. 2003 ;37:19-26
6. Chu CJ, Lok ASF. Clinical significance of hepatitis B virus genotypes. Hepatology. 2002 ;35:1274-1276
7. Chu CJ, Keeffe EB, Han SY. et al. Prevalence of HBV precore/core promoter variants in the United States. Hepatology. 2003 ;38:619-628
8. Perrillo RP. Acute flares in chronic hepatitis B: The Natural and unnatural history of an immunologically mediated liver disease. Gastroenterology. 2001 ;120:1009-1022
9. Fattovich G. Natural history of hepatitis B. Journal of Hepatology. 2003 ;39:S50-S58
10. McMahon BJ, Alberts SR, Wainwright RB. et al. Hepatitis B-related sequelae. Prospective study in 1400 hepatitis B surface antigen-positive Alaska Native carriers. Arch Intern Med. 1990 ;150:1051-4
11. Merican I, Guan R, Amarapuka D. et al. Chronic hepatitis B virus infection in Asian countries. Journal of Gastroenterology and Hepatology. 2000 ;15:1356-1361
12. Hoofnagle JH. Serologic markers of hepatitis B virus infection. Annu Rev Med. 1981 ;32:1-11
13. Ribeiro RM, Lo A, Perelson AS. et al. Dynamics of hepatitis B virus infection. Microbes Infect. 2002 ;4:829-35
14. Lok ASF, Lai CL, Wu PC. et al. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology. 1987 ;92:1839-43
15. Bortolotti F, Cadrobbi P, Crivellaro C. et al. Long-term outcome of chronic type B hepatitis in patients who acquire hepatitis B virus infection in childhood. Gastroenterology. 1990 ;99:805-10
16. Funk ML, Rosenberg DM, Lok ASF. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002 ;9:52-61
17. Keefe Emmet, Dieterich Douglas, Steve-Huy B. et al. A treatment Algorithm for the management of Chronic Hepatitis B Virus Infection in the United States. Clinical Gastroenterology and Hepatology. 2004 ;2:87-106
18. Papatheodoridis GV, Manesis E, Hadziyannis SJ. et al. The long-term outcome of interferon-Alpha treated and untreated patients with HbeAg-negative chronic hepatitis B. J Hepatol. 2001 ;34:306-13
19. Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2001 ;34:1225-41
20. Hsu YS, Chien RN, Yeh CT. et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002 ;35:1522-7
21. De Franchis R, Meuccie G, Vecchi M. et al. The Natural history of asymptomatic hepatitis B surface antigen carriers. Ann Intern Med. 1993 ;118:191-4
22. Bellentani S, Dal Molin G, Miglioli L. et al. Natural history of HBV infection: a 9 years follow-up of the Dionysos cohort. J Hepatol. 2002 ;36(Suppl. 1):228-
23. Rossi G, Pelizzari A, Motta M. et al. Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HBsAg carriers with lymphoid malignancies treated with chemotherapy. Br J Hematol. 2001 ;115:58-62
24. Chan TM, Fang GX, Tang CSO. et al. Preemptive lamivudine therapy based on HBV DNA level in HBsAg-positive kidney allograft recipients. Hepatology. 2002 ;36:1245-52
25. de Jongh FE, Janssen HL, de Man RA. et al. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of liver. Gastroenterology. 1992 ;103:1630-1635
26. Fattovich G, Giustina G, Schalm SW. et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. Hepatology. 1995 ;21:77-82
27. Kao JH, Chen PJ, Lai MY. et al. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000 ;118:554-9
28. Fattovich G, Pantalena M, Zagni I. et al. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort of 297 patients. Am J Gastroenterology. 2002 ;97:2886-95
29. Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988 ;61:1942-56
30. Benvegnue L, Fattovich G, Noventa F. et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. Cancer. 1994 ;74:2442-8
31. Tsai JF, Jeng JE, Ho MS, Chang WY. et al. Effect of hepatitis C and B virus infection on risk of hepatocellular carcinoma: a prospective study. Br J Cancer. 1997 ;76:968-74
32. Yang HI, Lu SN. et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002 ;347:168-174
Figures
Stages of HBV infection based on virus-host interaction. In persistent infected patients, the stages of immune tolerance and immune clearance clinically present as HBeAg positive chronic hapatitis B. The stage of inactive phase clinically presents as HBsAg carrier. * During the stage of reactivation, majority of patients remain HBeAg negative with positive HBeAb and their clinical presentation can be HBeAg negative chornic hepatitis B, but some patients may have seroreversion of HBeAg and present as HBeAg positive chornic hepatitis B.
Author biography
Calvin Q. Pan, MD, is a Faculty of Gastroenterology and Hepatology Fellowship Program at Mount Sinai School of Medicine in New York. He is also a Consultant Attending at Hepatology Services, Elmhurst Hospital Center of Mount Sinai Services, New York. His current researches include natural history and treatment of viral hepatitis. He currently works on investigating the association between hepatitis C virus and liver steatosis as well as hepatitis B treatment outcome studies.
Jin X. Zhang, MD, is Senior Fellow of Gastroenterology at the Division of Gastroenterology, Mount Sinai Hospital, Mount Sinai School of Medicine, New York. She is interested in viral hepatitis researches.
![]() Corresponding address:
Corresponding address:
Calvin Q. Pan, MD, Division of Gastroenterology and Hepatology, Department of Medicine, Elmhurst Hospital Center of Mount Sinai Services, 79-01 Broadway, Elmhurst, NY, 11373, Phone: 714-888-7728, Fax: 718-888-2147, Email: Calvinorg

 Global reach, higher impact
Global reach, higher impact