3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(3):181-192. doi:10.7150/ijms.1.181 This issue Cite
Research paper
Elevated plasma homocysteine is positively associated with age independent of C677T mutation of the methylenetetrahydrofolate reductase gene in selected Egyptian subjects
1 Departments of Chemical Pathology, Medical Research Institute Hospital, Alexandria University, Egypt.
2 Internal Medicine (Cardiology Unit), Medical Research Institute Hospital, Alexandria University, Egypt.
Received 2004-6-25; Accepted 2004-9-20; Published 2004-10-12
Abstract
This study aimed to evaluate the plasma homocysteine (tHcy) and folate levels as well as the methylenetetrahydrofolate reductase (MTHFR) C677T mutation in Egyptian subjects. Fasting total homocysteine (tHcy) and the (MTHFR) C677T mutation were evaluated in 50 healthy young control males (age 35-50 years, Gp1), 50 elderly males age ranged between 50-75 years without any cardiovascular diseases (Gp2) and 50 age matched elderly male patients (Gp3) with myocardial infarction. There was a significant elevation of plasma tHcy in the patients group and Gp2 compared to the young control group (Gp1). The total plasma homocysteine (tHcy) in the control group, Gp2 and the patients group were 17.99 ± 9.76, 39.9 ± 20.06 and 43.8 ± 13.13 μmol/L respectively. The frequency of the TT genotype was 12% in the patient group compared with 8 % in the young healthy controls and elderly subjects (Gp2). The CT genotype constituted 36%, 48% and 44% in the control group, Gp2 and the patients group respectively. There was no significant difference in the occurrence of the TT genotype between the studied groups. Plasma tHcy correlated positively with age, total cholesterol, urea, creatinine, glucose levels and carotid intimal thickness (CIT). Conclusion: The MTHFR mutation does not seem to be associated with either high tHcy or the occurrence of cardiovascular diseases in the studied patients. However, elevated plasma tHcy level positively correlates with age in the studied subjects.
Keywords: Homocysteine, MTHFR, coronary heart disease
1. Introduction
Homocysteine lies at an important metabolic branch point of methionine metabolism, between the remethylation and transsulfuration pathways [1 ]. These lead to the formation of methionine and cystathionine, respectively [2,3 ]. Several enzymes regulate these pathways under normal conditions [4 -6 ]. Methionine formation is tightly tied to a vitamin B12-dependent enzyme [4 ,7,8 ] methionine synthase, which uses 5-methyltetrahydrofolate as a carbon donor [6 ] . This donor is synthesized by the methylenetetrahydrofolate reductase (MTHFR) gene from 5,10-methylene-tetrahydrofolate [9,10 ]. Reduction in the activity of these enzymes caused by congenital defects and/or deficiencies in folate may affect the normal homocysteine pathway [9, 11 ].
A relationship between hyperhomocystinemia and cardiovascular disease is well established [12 -15 ]. A thermolabile variant of MTHFR, with reduced specific activity has been described [16 ]. Frosst et al [17 ] identified that a substitution of cytosine (C) by thymine (T) at nucleotide 677 of the MTHFR gene that converts an alanine to a valine residue was responsible for the thermolability of MTHFR. Actually, the frequency of the mutated allele was quite high, depending on the ethnic group analyzed [18 -21 ]. Some authors suggested that the homozygosity for the MTHFR C677T polymorphism was associated with an increased risk of coronary heart disease [9,22 ] whereas others failed to demonstrate this association [23-25 ]. Furthermore, a recent meta-analysis study pointed at a moderate increase in plasma homocysteine and the risk of CVD in patients with MTHFR mutation [26].
The tHcy level is strongly dependent on the folate and vitamin B12 status of each individual [27 ,11,28 ]. Plasma tHcy may be increased in cases of severe, folate deficiency [8 ] and folate supplements reduce plasma tHcy levels [29 ]. The response to folate supplements is affected by the number of the 677T alleles in the MTHFR gene, with the strongest response in 677T homozygotes [30 ]. On the other hand, the association between carotid and coronary artery disease is well recognized [31 ] and ultrasonography of the carotid arteries with measurement of the carotid intimal thickness (CIT) had been shown to correlate with the severity of atherosclerosis [31 ]. Some studies showed a strong correlation between elevated plasma homocysteine, MTHFR mutation and CIT [32,33] while others gave equivocal results [34,35].
No data are available about the frequency of the MTHFR mutation and its relation to tHcy in the Egyptian population. Thus the aim of the present study was to determine tHcy level and its relationship to MTHFR C677T polymorphism in patients with established coronary heart disease, age and sex matched elderly subjects and in healthy young controls.
2. Material and methods
Subjects
The current study was carried on three groups. A control group (n=50) consisted of healthy young male volunteers, their age ranged between 35-50 years (Gp1). Another group of healthy elderly subjects not complaining of any cardiovascular disease their age ranged between 50-75 years (Gp2). The patients group (Gp3) consisted of a total of 50 unrelated male patients with past history of myocardial infarction (age ranged between 50-75 years). All patients and controls were living in the same geographic area of Northern Egypt (Alexandria), all of them had the same life style (non of them was heavy smoker, had excessive alcohol consumption or special dietary habit). The exclusion criteria for all groups were as follows: vitamins supplementation (e.g. betaine, choline, folate, vitamin B6, or vitamin B12), the presence of any form of cancer, liver disease, primary renal disease or any collagenic diseases.
All subjects gave informed consent before the study began. Patients who had history of myocardial infarction (confirmed by ECG findings and elevated troponin T) were included in the study. All patients and controls had a full clinical examination, including history taking, blood pressure measurement and carotid ultrasonography for measurement of the carotid artery intimal thickness to assess the degree of atherosclerosis [36 ]. This was done using the ultrasonic machine TOSHIBA core-vision equipped with high frequency linear array transducer. The examination was done for the right and left common carotid arteries and the mean values of the two sites were used in the analysis. The transducer was positioned over the common carotid artery screening it from the origin up to the carotid bifurcation looking for any changes, plaque formation with measurement of the vessel thickness. Measurement of the CIT was done at the far wall of the vessel 1cm proximal to its bifurcation. Mean CIT was calculated as the average of five measurements to each common carotid artery [36 ].
Blood Collection
All blood samples were collected after an overnight fast (>10 hours) by venipuncture into an EDTA containing tube. A separated aliquot was kept for DNA extraction. Plasma samples were obtained by double centrifugation at room temperature for 15 minutes at 2000g. The plasma aliquots were immediately frozen at -70°C until use.
Laboratory measurements
Biochemical investigations including blood glucose, cholesterol, triglycerides, high density lipoprotein, urea and creatinine were carried out using Kone Lab. auto-analyzer. Plasma tHcy was determined by immunoassay (Abbott Laboratories, North Chicago, IL, U.S.A.). Plasma folate levels were measured by radioimmunoassay using a commercial kit (Dualcount Charcoal Boil Assay, Diagnostic Products Corporation, Los Angeles, CA).
MTHFR mutation analysis
DNA Extraction:
Genomic DNA was isolated from nucleated blood cells using a phenol chloroform method [37 ]. DNA samples were kept at -80ºC till analysed.
PCR-RFLP analysis
The C677T MTHFR gene mutation was detected by PCR-RFLP analysis using Hinf I restriction analysis of a 198-bp polymerase chain reaction–amplified fragment in the gene for MTHFR, according to Frosst et al [17 ]. Briefly, about 50 to 80 ng DNA samples were amplified in a final volume of 25 µL containing 1×PCR buffer with 1.5 mmol/L MgCl2, 2 unit Taq DNA polymerase, 100 µmol/L dNTP, and 0.5 µmol/L of each primer (5'-TGAAGGAGAAGGTGTCTGCGGGA-3' and 5'-AGGACGGTGCGGTGAGAGTG-3'). PCR was performed in a GeneAmp, thermocycler (Biorad, USA), and the profile consisted of an initial melting step of 2 min at 94°C; followed by 35 cycles of 30 s at 94°C, 30 s at 61°C, and 30 s at 72°C; and a final elongation step of 7 min at 72°C.
The restriction enzyme Hinf I (Promega, UK) was used to distinguish the C677T polymorphism, and the gain of a Hinf I restriction site occurs in the polymorphic allele. The wild genotype (677C) has a single band representing the entire 198-bp fragment, and the heterozygous genotype (677T) results in three fragments of 198, 175 and 23 bp, while the homozygous for the MTHFR mutation results in 2 fragments 175 and 23 bp. Finally the products of the Hinf I digestion were electrophoresed on 3% agarose gel.
To ensure quality control, genotyping was performed with blinding to case/control status, and random samples of cases and controls were tested twice by different persons, and the results were concordant for all masked cases.
Statistical analysis:
The distributions of plasma tHcy concentrations were positively skewed; therefore, they were transformed logarithmically to approximate normal distribution, and such data were analysed statistically. Statistical analysis of the data analysis was carried out using the ANOVA test. Prevalence of alleles and genotype among cases and control subjects were counted and compared with Hardy–Weinberg predictions [38 ]. Chi-square test (χ 2, Fisher's exact test) was used to test the distribution of the different genotypes in the different groups. For correlation studies, Pearson correlation test was used. P value of < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 10 statistical Package.
3. Results
The results of some clinical data and biochemical results of the different groups included in this study are shown in table I. Results are expressed as the (range) mean ± SD. As expected there was a significant increase in the blood pressure level with age which was especially noted in the patient group (age ranged between 50 and 75 years). The mean values of the carotid intimal thickness (CIT) were significantly higher in the patients group (Gp3) and the elderly healthy control group (Gp2) compared with the healthy young control group (Gp1). As regard the glycemic state, there was a significant increase in the blood glucose level both fasting and post-prandial in the patients group (Gp3) compared with Gp1 and Gp2. Also total cholesterol and creatinine were significantly higher in the patient group compared with the control and Gp2 (P< 0.05).
Plasma folate showed no significant difference between the patients group and the other two groups. There was poor correlation between the plasma tHcy and folate level.
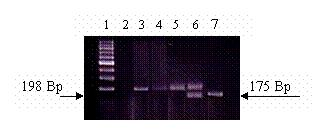
Figure 1 shows an agarose gel illustrating the different genotypes of the C667T mutation. The wild type CC shows a single band at the 198 base pair (Bp). The heterozygote CT showed two bands, one at the 198 and the other at the 175 base pair respectively (the third band of 23 base pairs could not be visualized on the agarose gel). The homozygote TT showed a single band at the 175 Bp. Table II shows the frequency of each genotype in the three studied groups and the results of the Chi square testing comparing each genotype in the three different groups. There was no significant difference in the occurrence of the TT genotype in any of the studied groups (P>0.05). Also the prevalence of the different genotypes did not deviate from the Hardy–Weinberg equilibrium for the control group, Gp2 or patients group.
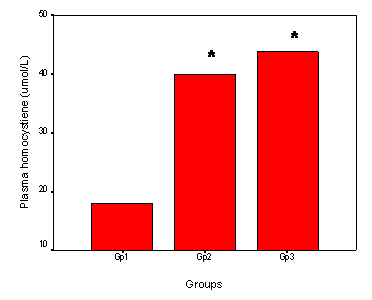
As regard the plasma tHcy, the means ± SD of Gp1, GP2 and GP3 were 17.99 ± 9.76, 39.9 ± 20.06 and 43.8 ± 13.13 μmol/L respectively. There was a significant elevation of plasma tHcy in the patient group (Gp3) and healthy elderly (Gp2) compared to the control group (Gp1) (Figure 2).
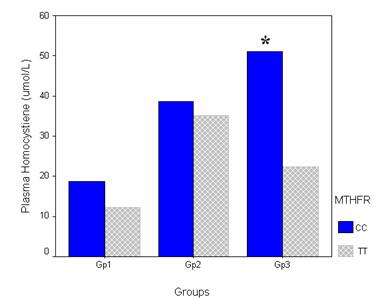
Plasma tHcy levels were compared in the CC and TT genotypes in the different groups to investigate the effect of the mutant allele (T). There was no significant difference regarding the tHcy between the CC and TT genotypes in Gp1 or Gp2. The CC genotype had a plasma tHcy of 18.74 ± 11.26 and 38.55 ± 22.63 μmol/L, while the TT genotype had a plasma tHcy of 12.2 ± 4.58 and 36.25 ± 8.96 in Gp1 and Gp2 respectively. On the other hand, in the patients group the CC genotype had an even significantly higher plasma tHcy when compared with the TT genotype (P<0.05). The CC and the TT genotypes had plasma tHcy of 51.09 ± 28.37 and 22.33 ± 6.71 respectively. (Figure 3)
When plasma tHcy was correlated with age, there was a significant positive correlation. The correlation coefficient value was 0.564. There was also a significant positive correlation between the level of the tHcy and the total cholesterol, urea, creatinine and the fasting and postprandial blood glucose. The correlation was significant at the 0.01 level.
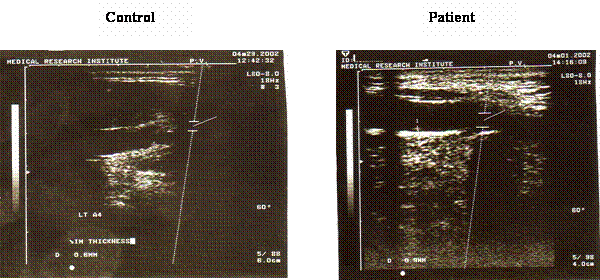
Figure 4 shows the result of the carotid ultrasonography in a patient with coronary heat disease and a young control subject. There was an apparent thickness of the carotid intima in the patient ultrasonography when compared with the control. Furthermore, there was a weak positive correlation between the CIT and the tHcy level, the correlation coefficient was 0.34.
4. Discussion
The principle findings of this study include the followings: first MTHFR gene mutation does not seem to be associated with the occurrence of coronary heart disease or the elevation of plasma tHcy in the studied patients. Plasma tHcy was significantly higher in elder patients with cardiovascular complications compared with the young control group. There was a significant positive correlation between plasma tHcy and age. Also, the high plasma tHcy was associated with the presence of mildly elevated serum urea and creatinine and poor glycemic control in the patients group. Moreover, the tHcy level correlated positively with the total serum cholesterol.
In this study, plasma homocysteine in the young control group was higher than the reference ranges provided by other studies performed at different ethnic populations [8,14,24]. This might represent an ethnic difference in plasma homocysteine and warrants a reference range study in the Egyptian population. The significantly higher plasma tHcy in the patients group compared with the young control group (Gp1) falls in line with the results of several previous studies which highlighted the causal relationship between the occurrence of the coronary heart diseases and the high plasma tHcy [14, 3 9, 40]. This finding might explain the higher degree of CIT in Gp2 and Gp3 compared to Gp1 (young healthy controls). It is hypothesized that the high tHcy level induces procoagulative effects that result in endothelial damage [41,42 ] that could precipitate the rupture of an established atherosclerotic plaque [25, 41,43].
Plasma tHcy although affected by several factors that control the metabolism of homocysteine pathway [4 4, 45 ] the most important determinant of plasma homocysteine is the folate intake [46,47 ]. Several previous reports illustrated the positive effect of folate intake on the reduction of plasma tHcy level and subsequent decrease in the incidence of cardiovascular diseases [46-49 ]. In the present study there was no significant difference between the different groups included in this study regarding the folate level. Also, there was no correlation between the plasma tHcy and the folate level. This finding although surprising, it is in accordance with the result of Verhoef et al [50 ] who found no significant difference in the plasma folate level between patients with vascular diseases and controls[50 ]. The lack of significant difference regarding the folate level in the studied groups might be explained by the high level of fruits and green vegetables in the diet of the Egyptian population in general. However, the lack of correlation between the plasma tHcy and the folate could be explained by the presence of mild renal impairment in the patients group, as evidenced by the significantly higher level of urea and creatinine in the patients group compared with the other two groups. This mild renal impairment might cause a resistant hyperhomocysteinaemia [51 ]despite the high folate intake as discussed below.
As regard the relationship between tHcy and MTHFR mutation, in this study, we did not find a significant correlation between high tHcy levels and MTHFR genotypes in our patients. This finding is in keeping with a recent report [52 ] which found no association between the plasma tHcy and the MTHFR mutation, although in a meta-analysis, Klerk et al [53 ] found that the MTHFR 677TT genotype was associated with elevated tHcy level. However, most studies did not show an association between the MTHFR mutation and subsequent development of cardiovascular diseases [23,24,54 ]. Our results are in keeping with the results of these studies.
Since Frosste et al [17 ] suggested the C667T mutation as a possible reason for the occurrence of vascular complications; several groups have evaluated this hypothesis in cardiovascular patients from different ethnic groups [19, 21, 55 ].
In the current study there was no significant difference between the patients group and the other two groups regarding the distribution of the TT genotype. These results are close to the results of previous studies that evaluated the TT genotype distribution in other ethnic populations [51,5 6, 5 7].
In the current study, when cases were stratified according to expression of the T allele and reanalyzed for the plasma tHcy, there was no significant increase in tHcy in the TT genotype individuals in the different groups. This finding supports more the lack of association between the presence of the T allele and the high tHcy levels in our patients. This could be explained by the presence of other factors that control tHcy [58,59 ] especially plasma folate and vitamin B12[30 ]. Furthermore the presence of other DNA mutations that could affect the homocysteine metabolism (e.g. A1298C mutation) [60 ]. Another reason might be the relatively small number enrolled in our study.
The current study showed a positive correlation between tHcy and age which is in keeping with previous results in different ethnic population [24, 60, 62, 63 ]. This could be explained by the presence of other factors that raise plasma tHcy with age, especially the increased deterioration in other organ functions. The later was reflected in the present study by the higher incidence of impaired glycemic control and hypertension. Both have adverse effect on the renal function. Furthermore, previous reports found an elevated tHcy in patients with renal impairment and insulin resistance [64-67 ]. Another possible reason may be the generalized slow down of some biological processes in the body with aging including methionine metabolism [61 ].
The current study showed a significantly higher plasma glucose level both fasting and post-prandial in the patient group and Gp2 compared with the control. This impairment in the glycemic control most probably reflects a state of insulin resistance. Elevated homocysteine is associated with several cardiovascular disease risk factors including endothelial dysfunction and abnormalities of clotting functions, which are also common features of insulin resistance syndrome [65 ]. Several studies have shown that the increase in insulin resistance was paralleled by a concomitant elevation of the plasma tHcy [66-68 ]. Furthermore, it has been demonstrated in rat model that hyperinsulinaemia suppresses hepatocyte expression of cystathionine beta-synthase enzyme [69 ] with subsequent development of hyperhomocysteinaemia [69 ].
This study also showed a positive correlation between the urea, and creatinine levels and plasma tHcy. The noticed impairment in the renal function is probably a complication of the cardiovascular disease itself (e.g. hypertension or heart failure) as patients with primary renal disease were not included in this study. This is in keeping with the results of several authors who reported an association between the impairment in renal functions and hyperhomocysteinaemia [70-72 ]. Moreover, Jager et al [73 ] showed that hyperhomocysteinaemia is an independent determinant of the development of microalbuminuria among non-diabetic subjects and hypothesized that; tHcy may play a pathophysiological role in the development of microalbuminuria [73 ]. The hyperhomocysteinaemia in patients with renal impairment was attributed to loss of renal homocysteine uptake and metabolism [74 ] which normally accounts for about 70% of daily homocysteine removal from the plasma [74, 75 ]. Thus elimination of a substantial homocysteine metabolizing capacity of normal kidneys could account for the presence of this refractory high homocysteine despite the high folate level in the patients group [76 ]
In the current study, there was a significantly higher serum cholesterol level in the patient group when compared with the other two groups. Also, this high cholesterol correlated positively with the tHcy level. This high cholesterol might be attributed to increase in weight gain with age, lack of physical activity or improper life style with high intake of fatty foods. However, the association of high homocysteine found in the current study and the cholesterol level could be explained by causal relationship between plasma tHcy and cholesterol.
Li H et al [77 ] demonstrated that high plasma tHcy has a positive effect on the hydroxyl methyl glutaryl -CoA synthase enzyme ( the rate limiting enzyme in cholesterol biosynthesis) and hence hypercholesterolaemia. Furthermore, they suggested that elevated homocysteine increases the cholesterol accumulation in the endothelial cells and suggested the use of statins in patients with elevated homocysteine even without hypercholesterolaemia.
In conclusion this study has shown a positive association between elevated plasma tHcy and age. There was no association between the MTHFR mutation and either the presence of coronary heart disease or the high plasma tHcy in our patients. Furthermore, there was a positive correlation between the plasma tHcy and impairment of both renal functions and glycemic control.
However, the relationship between plasma tHcy and other mutations in the MTHFR gene such as the A1298C and the degree of insulin resistance in the studied groups remains to be addressed. Furthermore, a recent report [67 ] stressed on the crucial role played by cytokines in the evolution of insulin resistance. Could hyperhomocysateineamia be a result of derangement of certain cytokine production?
Abbreviations
tHcy: total homocysteine; MTHFR: methylenetetrahydrofolate reductase; Gp: Group; CIT: Carotid intimal thickness
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999 ;19 :217 -46
2. Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992 ;55 (1) :131 -8
3. Ubbink JB, Vermaak WJ, van der Merwe A, Becker PJ. Vitamin B-12, vitamin B-6, and folate nutritional status in men with hyperhomocysteinemia. Am J Clin Nutr. 1993 ;57 (1) :47 -53
4. Mason JB, Miller JW. The effects of vitamins B12, B6, and folate on blood homocysteine levels. Ann N Y Acad Sci. 1992 ;669 :197 -203
5. Choumenkovitch SF, Selhub J, Bagley PJ, Maeda N, Nadeau MR, Smith DE, Choi SW. In the cystathionine beta-synthase knockout mouse, elevations in total plasma homocysteine increase tissue S-adenosylhomocysteine, but responses of S-adenosylmethionine and DNA methylation are tissue specific. J Nutr. 2002 ;132 (8) :2157 -60
6. Dudman NP, Guo XW, Gordon RB, Dawson PA, Wilcken DE. Human homocysteine catabolism: three major pathways and their relevance to development of arterial occlusive disease. J Nutr. 1996 ;126 (4 Suppl) :1295S -300S
7. Miller JW, Nadeau MR, Smith J, Smith D, Selhub J. Folate-deficiency-induced homocysteinaemia in rats: disruption of S-adenosylmethionine's co-ordinate regulation of homocysteine metabolism. Biochem J. 1994 ;298 ( Pt 2) :415 -9
8. Dalery K, Lussier-Cacan S, Selhub J, Davignon J, Latour Y, Genest J. Homocysteine and coronary artery disease in French Canadian subjects: relation with vitamins B12, B6, pyridoxal phosphate, and folate. Am J Cardiol. 1995 ;75 (16) :1107 -11
9. Kluijtmans LA, van den Heuvel LP, Boers GH, Frosst P, Stevens EM, van Oost BA, den Heijer M, Trijbels FJ, Rozen R, Blom HJ. Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet. 1996 ;58 (1) :35 -41
10. Lievers KJ, Boers GH, Verhoef P, den Heijer M, Kluijtmans LA, van der Put NM, Trijbels FJ, Blom HJ. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med. 2001 ;79 (9) :522 -8
11. Ubbink JB. Should all elderly people receive folate supplements? Drugs Aging. 1998 ;13 (6) :415 -20
12. Kosokabe T, Okumura K, Sone T, Kondo J, Tsuboi H, Mukawa H, Tomida T, Suzuki T, Kamiya H, Matsui H, Hayakawa T. Relation of a common methylenetetrahydrofolate reductase mutation and plasma homocysteine with intimal hyperplasia after coronary stenting. Circulation. 2001 ;103 (16) :2048 -54
13. Albert CM, Rifai N, Stampfer MJ, Ridker PM. Prospective Study of C-Reactive Protein, Homocysteine, and Plasma Lipid Levels as Predictors of Sudden Cardiac Death. Circulation. 2002 ;105 (22) :2595 -9
14. Sundstrom J, Sullivan L, D'Agostino RB, Jacques PF, Selhub J, Rosenberg IH, Wilson PW, Levy D, Vasan RS. Plasma Homocysteine, Hypertension Incidence, and Blood Pressure Tracking: The Framingham Heart Study. Hypertension. 2003 ;42 (6) :1100 -5
15. Adachi H, Hirai Y, Fujiura Y, Matsuoka H, Satoh A, Imaizumi T. Plasma homocysteine levels and atherosclerosis in Japan: epidemiological study by use of carotid ultrasonography. Stroke. 2002 ;33 (9) :2177 -81
16. Kang SS, Passen EL, Ruggie N, Wong PW, Sora H. Thermolabile defect of methylenetetrahydrofolate reductase in coronary artery disease. Circulation. 1993 ;88 (4 Pt 1) :1463 -9
17. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 ;10 (1) :111 -3
18. Ubbink JB, Christianson A, Bester MJ, Van Allen MI, Venter PA, Delport R, Blom HJ, van der Merwe A, Potgieter H, Vermaak WJ. Folate status, homocysteine metabolism, and methylene tetrahydrofolate reductase genotype in rural South African blacks with a history of pregnancy complicated by neural tube defects. Metabolism. 1999 ;48 (2) :269 -74
19. Mogk RL, Rothenmund H, Evans JA, Carson N, Dawson AJ. The frequency of the C677T substitution in the methylenetetrahydrofolate reductase gene in Manitoba. Clin Genet. 2000 ;58 (5) :406 -8
20. Hanson NQ, Aras O, Yang F, Tsai MY. C677T and A1298C polymorphisms of the methylenetetrahydrofolate reductase gene: incidence and effect of combined genotypes on plasma fasting and post-methionine load homocysteine in vascular disease. Clin Chem. 2001 ;47 (4) :661 -6
21. Esfahani ST, Cogger EA, Caudill MA. Heterogeneity in the prevalence of methylenetetrahydrofolate reductase gene polymorphisms in women of different ethnic groups. Am Diet Assoc. 2003 ;103 (2) :200 -7
22. Christensen B, Frosst P, Lussier-Cacan S, Selhub J, Goyette P, Rosenblatt DS, Genest J Jr, Rozen R. Correlation of a common mutation in the methylenetetrahydrofolate reductase gene with plasma homocysteine in patients with premature coronary artery disease. Arterioscler Thromb Vasc Biol. 1997 ;17 (3) :569 -73
23. Brugada R, Marian AJ. A common mutation in methylenetetrahydrofolate reductase gene is not a major risk of coronary artery disease or myocardial infarction. Atherosclerosis. 1997 ;128 (1) :107 -12
24. Thogersen AM, Nilsson TK, Dahlen G, Jansson JH, Boman K, Huhtasaari F, Hallmans G. Homozygosity for the C 677 ->T mutation of 5,10-methylenetetrahydrofolate reductase and total plasma homocyst(e)ine are not associated with greater than normal risk of a first myocardial infarction in northern Sweden. Coron Artery Dis. 2001 ;12 (2) :85 -90
25. Wilcken DE, Wang XL, Wilcken B. Methylenetetrahydrofolate reductase (MTHFR) mutation, homocysteine, and coronary artery disease. Circulation. 1997 ;96 (8) :2738 -40
26. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002 ;325 (7374) :1202 -
27. Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: Probable benefits of increasing folic acid intakes. JAMA. 1994 ;274 (13) :1049 -57
28. Ubbink JB, van der Merwe A, Vermaak WJ, Delport R. Hyperhomocysteinemia and the response to vitamin supplementation. Clin Investig. 1993 ;71 (12) :993 -8
29. Chauveau P, Chadefaux B, Coude M, Aupetit J, Kamoun P, Jungers P. Long-term folic acid (but not pyridoxine) supplementation lowers elevated plasma homocysteine level in chronic renal failure. Miner Miner Electrolyte Metab. 1996 ;22 (1-3) :106 -9
30. Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999 ;340 (19) :1449 -54
31. Kato M, Habara S, Takemoto H, Goto K, Nakaoka K. Clinical implications of carotid artery remodeling in acute coronary syndrome: ultrasonographic assessment of positive remodeling. J Am Coll Cardiol. 2003 ;42 (6) :1026 -32
32. Passaro A, Vanini F, Calzoni F, Alberti L, Zamboni PF, Fellin R, Solini A. Plasma homocysteine, methylenetetrahydrofolate reductase mutation and carotid damage in elderly healthy women. Atherosclerosis. 2001 ;157 (1) :175 -80
33. Ravera M, Viazzi F, Berruti V, Leoncini G, Zagami P, Bezante GP, Rosatto N, Ravazzolo R, Pontremoli R, Deferrari G. 5,10-Methylenetetrahydrofolate reductase polymorphism and early organ damage in primary hypertension. Am J Hypertens. 2001 ;14 (4 Pt 1) :371 -6
34. Scaglione L, Gambino R, Rolfo E, Lillaz E, Gai M, Cassader M, Pagano G, Cavallo-Perin P. Plasma homocysteine, methylenetetrahydrofolate reductase gene polymorphism and carotid intima-media thickness in Italian type 2 diabetic patients. Eur J Clin Invest. 2002 ;32 (1) :24 -8
35. McQuillan BM, Beilby JP, Nidorf M, Thompson PL, Hung J. Hyperhomocysteinemia but not the C677T mutation of methylenetetrahydrofolate reductase is an indepedent risk determinant of carotid wall thickening: The Perth Carotid Ultrasound Disease Assessment Study (CUDAS). Circulation. 1999 ;99 (18) :2383 -8
36. Takiuchi S, Fujii H, Kamide K, Horio T, Nakatani S, Kawano Y, Higaki J, Ogihara T. Carotid intima-media thickness is correlated with impairment of coronary flow reserve in hypertensive patients without coronary artery disease. Hypertens Res. 2003 ;26 (12) :945 -51
37. Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols In Molecular Biology, 2nd ed. New York: Wiley Interscience. 1987
38. Emery AEH. Hardy-Weinberg equilibrium and the estimation of gene frequencies. In: (ed.) Emery AEH. Methodology in medical genetics: an introduction to statistical methods. Edinburgh: Churchill Livingstone. 1976 :3- - 9
39. Hoogeveen EK, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Hyperhomocysteinemia increases risk of death, especially in type 2 diabetes: 5-year follow-up of the Hoorn Study. Circulation. 2000 ;101 (13) :1506 -11
40. Brattstrom L, Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am J Clin Nutr. 2000 ;72 (2) :315 -23
41. Wang X. A theory for the mechanism of homocysteine-induced vascular pathogenesis. Med Hypotheses. 1999 ;53 (5) :386 -94
42. Munshi M, Stone A, Fink L, Fonseca V. Hyperhomocysteinemia following a methionine load in patients with non-insulin-dependent diabetes mellitus and macrovascular disease. Metabolism. 1996 ;45 (1) :133 -5
43. Hoogeveen EK, Kostense PJ, Beks PJ, Mackaay AJ, Jakobs C, Bouter LM, Heine RJ, Stehouwer CD. Hyperhomocysteinemia is associated with an increased risk of cardiovascular disease, especially in non-insulin-dependent diabetes mellitus: a population-based study. Arterioscler Thromb Vasc Biol. 1998 ;18 (1) :133 -8
44. Jang Y, Lee JH, Kim OY, Park HY, Lee SY. Consumption of Whole Grain and Legume Powder Reduces Insulin Demand, Lipid Peroxidation, and Plasma Homocysteine Concentrations in Patients With Coronary Artery Disease: Randomized Controlled Clinical Trial. Arterioscler Thromb Vasc Biol. 2001 ;21 (12) :2065 -71
45. Seshadri N, Robinson K. Homocysteine and coronary risk. Curr Cardiol Rep. 1999 ;1 (2) :91 -8
46. Selhub J, Jacques PF, Bostom AG, Wilson PW, Rosenberg IH. Relationship between plasma homocysteine and vitamin status in the Framingham study population. Impact of folic acid fortification. Public Health Rev. 2000 ;28 (1-4) :117 -45
47. Bostom AG. Folic acid fortification of food. JAMA. 1996 ;275 (9) :681 -3
48. Bostom AG, Selhub J, Jacques PF, Rosenberg IH. Power Shortage: clinical trials testing the "homocysteine hypothesis" against a background of folic acid-fortified cereal grain flour. Ann Intern Med. 2001 ;135 (2) :133 -7
49. Kim MK, Ordovas JM, Selhub J, Campos H. B vitamins and plasma homocysteine concentrations in an urban and rural area of Costa Rica. J Am Coll Nutr. 2003 ;22 (3) :224 -31
50. Verhoef P, Kok FJ, Kruyssen DA, Schouten EG, Witteman JC, Grobbee DE, Ueland PM, Refsum H. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1997 ;17 (5) :989 -95
51. Brosnan JT, Hall B, Selhub J, Nadeau MR, Bostom AG. Renal metabolism of homocysteine in vivo. Biochem Soc Trans. 1995 ;23 (3) :470S -
52. Morelli VM, Lourenco DM, D'Almeida V, Franco RF, Miranda F, Zago MA, Noguti MA, Cruz E, Kerbauy J. Hyperhomocysteinemia increases the risk of venous thrombosis independent of the C677T mutation of the methylenetetrahydrofolate reductase gene in selected Brazilian patients. Blood Coagul Fibrinolysis. 2002 ;13 (3) :271 -5
53. Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002 ;288 (16) :2023 -31
54. Folsom AR, Nieto FJ, McGovern PG, Tsai MY, Malinow MR, Eckfeldt JH, Hess DL, Davis CE. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998 ;98 (3) :204 -10
55. Qi Z, Hoffman G, Kurtycz D, Yu J. Prevalence of the C677T substitution of the methylenetetrahydrofolate reductase (MTHFR) gene in Wisconsin. Genet Med. 2003 ;5 (6) :458 -9
56. Verhoef P, Kok FJ, Kluijtmans LA, Blom HJ, Refsum H, Ueland PM, Kruyssen DA. The 677C-->T mutation in the methylenetetrahydrofolate reductase gene: associations with plasma total homocysteine levels and risk of coronary atherosclerotic disease. Atherosclerosis. 1997 ;132 (1) :105 -13
57. Brattstrom L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation. 1998 ;98 (23) :2520 -6
58. Andersson A, Brattstrom L, Israelsson B, Isaksson A, Hamfelt A, Hultberg B. Plasma homocysteine before and after methionine loading with regard to age, gender, and menopausal status. Eur J Clin Invest. 1992 ;22 (2) :79 -87
59. Jacques PF, Bostom AG, Williams RR, Ellison RC, Eckfeldt JH, Rosenberg IH, Selhub J, Rozen R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation. 1996 ;93 (1) :7 -9
60. Friedman G, Goldschmidt N, Friedlander Y, Ben-Yehuda A, Selhub J, Babaey S, Mendel M, Kidron M, Bar-On H. A common mutation A1298C in human methylenetetrahydrofolate reductase gene: association with plasma total homocysteine and folate concentrations. J Nutr. 1999 ;129 (9) :1656 -61
61. Stampfer MJ, Grodstein F. Can homocysteine be related to physical functioning? Am J Med. 2002 ;113 (7) :610 -1
62. Ma J, Stampfer MJ, Hennekens CH, Frosst P, Selhub J, Horsford J, Malinow MR, Willett WC, Rozen R. Methylenetetrahydrofolate reductase polymorphism, plasma folate, homocysteine, and risk of myocardial infarction in US physicians. Circulation. 1996 ;94 (10) :2410 -6
63. Russo GT, Friso S, Jacques PF, Rogers G, Cucinotta D, Wilson PW, Ordovas JM, Rosenberg IH, Selhub J. Age and gender affect the relation between methylenetetrahydrofolate reductase C677T genotype and fasting plasma homocysteine concentrations in the Framingham Offspring Study Cohort. J Nutr. 2003 ;133 (11) :3416 -21
64. Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Insulin resistance is associated with elevated plasma total homocysteine levels in healthy, non-obese subjects. Atherosclerosis. 1998 ;139 (1) :197 -8
65. Bar-On H, Kidron M, Friedlander Y, Ben-Yehuda A, Selhub J, Rosenberg IH, Friedman G. Plasma total homocysteine levels in subjects with hyperinsulinemia. J Intern Med. 2000 ;247 (2) :287 -94
66. Fonseca VA, Fink LM, Kern PA. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis. 2003 ;167 (1) :105 -9
67. Benjafield AV, Wang XL, Morris BJ. Tumor necrosis factor receptor 2 gene (TNFRSF1B) in genetic basis of coronary artery disease. J Mol Med. 2001 ;79 (2-3) :109 -15
68. Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, D'Agostino RB Sr, Wilson PW. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care. 2001 ;24 (8) :1403 -10
69. McCarty MF. Insulin secretion as a potential determinant of homocysteine levels. Med Hypotheses. Med Hypotheses. 2000 ;55 (5) :454 -5
70. Robinson K, Dennis VW. From microalbuminuria to hyperhomocysteinemia. Kidney Int. 1998 ;54 (1) :281 -2
71. Bostom AG, Gohh RY, Bausserman L, Hakas D, Jacques PF, Selhub J, Dworkin L, Rosenberg IH. Serum cystatin C as a determinant of fasting total homocysteine levels in renal transplant recipients with a normal serum creatinine. J Am Soc Nephrol. 1999 ;10 (1) :164 -6
72. Robinson K. Renal disease, homocysteine, and cardiovascular complications. Circulation. 2004 ;109 (3) :294 -5
73. Jager A, Kostense PJ, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Donker AJ, Stehouwer CD. Serum Homocysteine Levels Are Associated With the Development of Microalbuminuria: The Hoorn Study. Arterioscler Arterioscler Thromb Vasc Biol. 2001 ;21 (1) :74 -81
74. Bostom AG, Lathrop L. Hyperhomocysteinemia in end-stage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int. 1997 ;52 (1) :10 -20
75. Guttormsen AB, Ueland PM, Svarstad E, Refsum H. Kinetic basis of hyperhomocysteinemia in patients with chronic renal failure. Kidney Int. 1997 ;52 (2) :495 -502
76. B Bostom AG, Shemin D, Verhoef P, Nadeau MR, Jacques PF, Selhub J, Dworkin L, Rosenberg IH. Elevated fasting total plasma homocysteine levels and cardiovascular disease outcomes in maintenance dialysis patients: A prospective study. Arterioscler Thromb Vasc Biol. 1997 ;17 (11) :2554 -8
77. Li H, Lewis A, Brodsky S, Rieger R, Iden C, Goligorsky MS. Homocysteine Induces 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Vascular Endothelial Cells: A Mechanism for Development of Atherosclerosis? Circulation. 2002 ;105 (9) :1037 -43
Tables and Figures
Some clinical and biochemical data of the different groups included in the study.
| Parameter | (Range) Mean ± SD | ||
|---|---|---|---|
| Controls (Gp1) (n:50) | Healthy elderly (Gp2) (n:50) | Patients (Gp3) (n:50) | |
| Age (years) | (35-50) 42.76 ± 5.24 | (50-75) 59.76 ± 8.45 | (50-75) 62.92 ± 7. 1 |
| SBP (mmHg) | (120 -149)134.6 ± 9.3 | (133-178) 154. 6 ±14.78* | (140-180)162.8 ±10.78* |
| DBP (mmHg) | (73 -93) 85.44 ± 5.4 | (74-94) 89.4 ± 5.43 | (88-110) 97.2 ± 6.85* |
| CIT (mm) | (0.3-0.64) 0.47 ± 0.103 | (0.7-1.2) 0.86 ± 0.14* | (0.7-1.6)1.03 ± 0.26* |
| FBG (mmol/L) | (4.1-6.1) 5.2 ± 0.58 | (4.3-6.9) 5.8 ± 1.27 | (4.1-7.7)6.04 ± 1.17* |
| PPBG (mmol/L) | (4.2-6.5) 5.13 ± 0.57 | (4.3-10.3) 6.2 ± 1.83 | (4.2-10.8)7.2 ± 2.34* |
| TC (mmol/L) | (3-5.2) 4.3 ± 0.46 | (3.4-6.8) 4.9 ± 1.14 | (3.2-7.6) 6.3 ±1.33* |
| TG (mmol/L) | (0.81-2.0) 1.4 ± 0.37 | (0.84-3.2)1.83 ± 0.81 | (0.94-3.1)1.93 ± 0.62* |
| HDL-C (mmol/L) | (0.83-1.45) 1.1 ±0.23 | (0.6-1.76) 1.2 ±0.31 | (0.83-1.32)1.1± 1.2 |
| Creatinine (μmol/L) | (79.6-109.6) 104 ±10.9 | (106-141.4)125.2 ±18.4* | (79.6-221)136.5 ± 44.4* |
| tHcy (μmol/L) | (5.1 -36) 17.99 ± 9.76 | (13-76) 39.9 ± 20.06* | (18-95) 43.8± 23.13* |
| Folate (nmol/L) | (6-37) 29.04 ±9.71 | (19-52) 31.52 ±7.51 | (7-49)30.52 ±11.51 |
Gp: group, SBP: systolic blood pressure, DBP: diastolic blood pressure, CIT: Carotid intimal thickness in millimeters, FBG, Fasting blood glucose, PPBG: Post-prandial blood glucose, TC: Total cholesterol, TG: triglycerides, HDL-C. High density lipoprotein cholesterol, tHcy: Total homocysteine.
*: Significant difference versus the young control group (Gp1). P<.005 was considered significant.
Frequency of the MTHFR genotypes and the result of the Chi square testing in the different studied groups. Showing the frequency and the results of the cross tabulation of the T allele and the different genotypes in the studied groups. The total number of cases was 50 in each group. There was no significant difference regarding the distribution of the CC, CT, TT genotypes in the three studied groups (P>0.05). Chi square test (Exact Fisher test) was used.
| Group | Frequency of the different MTHFR genotypes | Group | MTHFR Genotypes | Total | P Value | ||||
| CC% | CT% | TT% | CC | CT | TT | ||||
| Gp1 | 56 | 36 | 8 | Gp1 | 28 | 18 | 4 | 50 | |
| Gp2 | 44 | 48 | 8 | Gp2 | 22 | 24 | 4 | 50 | 0.347 |
| Gp3 | 44 | 44 | 12 | Gp3 | 22 | 22 | 6 | 50 | 0.230 |
| Total | 72 | 64 | 14 | 150 | |||||
Different MTHFR genotypes.3% agarose gel electrophoresis showing the different genotypes of the MTHFR mutation. DNA samples were amplified using PCR and digested with Hinf1 restriction enzyme. The presence of C to T mutation creates a Hinf1 restriction site. Lane 1: 100 base pairs (Bp) ladder marker. Lane 2: Negative control (no DNA was added.) Lane 3, 4, 5: Wild type CC genotype. Only one band could be seen at approximately the 198 Bp. Lane 6: CT heterozygous genotype. Two DNA fragments could be seen. The first at the 198 Bp, while the second at the 175 Bp. Lane 7: TT homozygous genotype. Only one band is seen at the approximately the 175 Bp.
Plasma homocysteine in the studied groups. Bar Chart illustrating the plasma homocysteine in the young control group (Gp1, age range 35-50 years) and the Gp2 (elderly control subjects aged between 50 and 75 years) and the patients group (Gp3, age range: 50-75 years). The number of subjects included was 50 in each group. * Significant difference versus (Gp1). P<0.05.
The effect of the T allele on the plasma homocysteine in the different studied groups. Bar chart illustrating the effect of the T allele on the level of homocysteine in the different groups. Cases were stratified according to the genotype into CC (wild type) and TT (mutant homozygous). MTHFR: Methylene tetrahydrofolate reductase. * Significant difference versus the TT genotype. P<0.05.
Ultrasonography for the carotid artery in a control subject and a patient with coronary heart disease. Carotid ultrasonography for measurement of the carotid artery intimal thickness (CIT) was done using the ultrasonic machine equipped with high frequency linear array transducer. The examination was done for the right and left common carotid arteries and the mean values of the two sites were used in the analysis. There is an apparent increase in the degree of carotid intimal thickness in the examined patient (0.9mm) compared with 0.6 mm in the control subject.
Author biography
Mohamed EL-SAMMAK obtained MSc (1998) and Ph.D (2001) in Clinical Laboratory Sciences from Nottingham University, UK, and MRCPath (2003) from Royal College UK. He currently works as a lecturer & honorary Consultant in Clinical Pathology at Alexandria University, Egypt. He is involved in several research projects such as the evaluation of methylenetetrahdrofolate reductase gene mutation in Egyptian patients with coronary heart disease; evaluation of the clinical utility of procalcitonin in tonsilopharingitis of various etiologies; ACE gene polymorphism in patients with pre-eclampsia.
Mona Kandil (MBCHB, MSc, PhD,MD) is a professor and head of Clinical Pathology department in Alexandria University, and her main research interest is Molecular Chemical Pathology.
Safaa El-Hifni (MBCHB, MSc, PhD, MD) is an emirates professor in Clinical Pathology at Alexandria University where she has been head of the department in Alexandria Medical Research Institute Teaching hospital till 2001. Her main research interest is analytical Chemical Pathology especially Chromatography and mass spectroscopy.
![]() Corresponding address:
Corresponding address:
Dr Mohamed Elsammak, Medical Research Institute hospital, 165 El-Horreya Street, Department of Chemical Pathology. POB: 21561 Alexandria, EGYPT. Email: myelsammakcom

 Global reach, higher impact
Global reach, higher impact