Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(3):170-180. doi:10.7150/ijms.1.170 This issue Cite
Review
A review of anatomical and mechanical factors affecting vertebral body integrity
1. Centre for Health, Exercise and Sports Medicine, School of Physiotherapy, University of Melbourne, Australia.
2. Department of Medicine, Royal Melbourne Hospital, University of Melbourne, Australia.
3. Institute of Medical and Veterinary Science, South Australia.
Received 2004-7-1; Accepted 2004-9-27; Published 2004-10-12
Abstract
Background: The aetiology of osteoporotic vertebral fracture is multifactorial and may be conceptualised using a systems framework. Previous studies have established several correlates of vertebral fracture including reduced vertebral cross-sectional area, weakness in back extensor muscles, reduced bone mineral density, increasing age, worsening kyphosis and recent vertebral fracture. Alterations in these physical characteristics may influence biomechanical loads and neuromuscular control of the trunk and contribute to changes in subregional bone mineral density of the vertebral bodies.
Methods: This review discusses factors that have received less attention in the literature, which may contribute to the development of vertebral fracture. A literature review was conducted using electronic databases including Medline, Cinahl and ISI Web of Science to examine the potential contribution of trabecular architecture, subregional bone mineral density, vertebral geometry, muscle force, muscle strength, neuromuscular control and intervertebral disc integrity to the aetiology of osteoporotic vertebral fracture.
Interpretation: A better understanding of factors such as biomechanical loading and neuromuscular control of the trunk may help to explain the high incidence of subsequent vertebral fracture after sustaining an initial vertebral fracture. Consideration of these issues may be important in the development of prevention and management strategies.
Keywords: osteoporosis, vertebral fracture, bone density, spinal biomechanics, neuromuscular control
1. Introduction
Osteoporosis is a metabolic bone disorder characterised by low bone mass and micro-architectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk [93]. Osteoporosis is increasingly recognised as an important public health problem due to the significant physical, economic and psychosocial ramifications of fracture. Vertebral fractures are a hallmark of osteoporosis and are associated with back pain, functional disability, reduced health-related quality of life and increased mortality. These associations become more significant with increasing numbers of vertebral fractures [24, 69].
Previous reviews have outlined the influence of bone mineral density (BMD), bone loss and important engineering principles such as bone geometry, mechanical properties and load transmission on the capacity of a vertebral body to resist physiologic loads that may contribute to vertebral fracture or deformity [32, 31, 65]. With advances in densitometric technologies and ex vivo analyses of trabecular bone properties, it is now evident that heterogeneity exists within the trabecular bone of a vertebral body. This observation may assist in the understanding of the complex aetiology underlying osteoporotic vertebral fracture. However, we postulate that trabecular bone properties cannot explain entirely why a difference in the prevalence rate of vertebral fractures exists among individuals with comparable BMD. It is likely that factors other than BMD operate to influence fracture risk, for example physiologic loading of vertebral bodies and the neuromuscular characteristics of trunk musculature.
Previously, the factors discussed have been viewed as mutually exclusive, but consideration of their potential interactions may assist understanding of the complex aetiology of these fractures. Therefore, the aim of this review is to highlight factors identified in the literature that may act to influence the risk of sustaining an osteoporotic vertebral fracture.
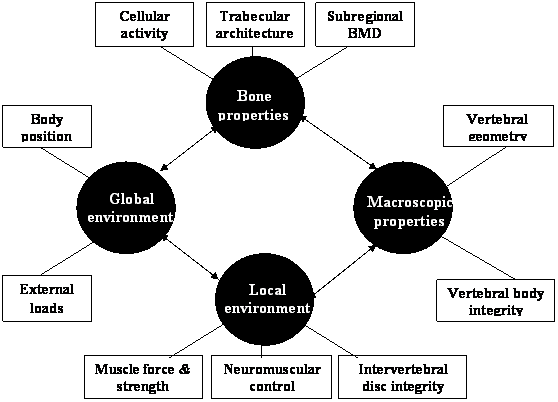
The risk of osteoporotic vertebral fracture can be conceptualised as a function of loading conditions and the ability of the human spine to withstand the load. Bone strength depends on its micro-architectural and macro-architectural features [80]. These features can be affected by the pathophysiology of osteoporosis and the local and global environment in which the bone operates. The local environment includes muscle and connective tissue attachments and the adjacent intervertebral disc, while the global environment can be considered to be the entire body operating in space (see figure 1).
Diagram of the systems approach that may be applied to explain the multifactorial aetiology underlying osteoporotic vertebral fracture. Bone, macroscopic, local and global factors may interact to influence the risk of sustaining a vertebral fracture.
2. Bone Structural Features
2.1. Trabecular micro-architecture
The design of the trabecular network allows for the efficient distribution of compressive and shear forces. This efficiency exists via the orientation and spacing of the three dimensional arrangement of osseous plates and struts that are continuous with the inner surface of the cortex. Vertically-orientated trabeculae attenuate axial forces, while horizontal plates respond to the tensile (shear) stresses transferred from the intervertebral disc [88]. An excess of bone resorption over formation leads to trabecular deterioration and loss. Deterioration in the trabecular architecture transforms the densely connected, plate-like trabeculae into discontinuous rod-like structures [1, 82]. Such transformations, if severe enough, are a hallmark of osteoporosis.
Trabecular bone strength is in part attributable to its large surface to volume ratio. This ratio is approximately four times greater than that observed in cortical bone [72]. With age and menopause, the trabecular bone mass in the vertebral body (centrum) declines at a faster rate than cortical bone mass. Cortical bone therefore dominates in the elderly spine. Generally, women loose 20% of peak cortical bone mass and 40-50% of trabecular mass by 90 years of age [58]. This results in a decline in the load-bearing capacity of the vertebral body. For example, load bearing capacity in a lumbar vertebra can decrease from 1000kg in a young individual to 60-90kg with age [62]. In osteoporosis, the normal age-related changes in cortical thickness and trabecular density become more pronounced resulting in a significant decline of compressive strength in the vertebral body, increasing the likelihood of fracture. A loss of trabecular bone in particular, will contribute significantly to a decline in mechanical strength of the vertebral body [94]. Ex vivo investigations demonstrate that mechanical characteristics of vertebral bone to strain is dominated by trabecular bone properties [43, 81].
2.2. Subregional Bone Mineral Density (srBMD)
The characteristic reduction in bone mass per unit of volume, i.e. volumetric BMD in osteoporosis interferes with the mechanical support properties of the bone. Various measures of BMD correlate strongly with compressive failure loads of vertebrae and can therefore be used as convenient and generally reliable indicators of compressive strength of vertebrae in vivo when measured with tools such as dual energy x-ray absorptiometry (DXA) and quantitative computed tomography (QCT) [31, 65]. Approximately 75-90% of the variance in bone strength is accounted for by apparent BMD [53, 87]. Low BMD is one of the most important risk factors for osteoporotic fracture [13]. However, examination of BMD alone is insufficient to explain the basis for vertebral fracture. Studies have shown differences in prevalence rates for fracture among individuals with comparable BMD [1, 12, 15, 18, 28, 46, 61, 73, 79, 89, 90]. These observations suggest that a single measure of BMD may not be representative of vertebral capacity and/or that other factors may operate independent of BMD measures. For example, risk variation between individuals may lie in the more subtle subregional density differences within the vertebral body. Subregional differences are hidden when whole vertebral body BMD is measured [46].
During aging, there is a non-uniform loss of bone within the vertebral body, resulting in trabecular bone density becoming non-homogenous throughout the centrum [2, 3, 56]. BMD therefore becomes disproportionate between different regions of the vertebral body. In an examination of cancellous bone morphometry of lumbar vertebrae, Simpson and colleagues [82] discovered that BMD, as measured by the ratio of bone volume to total volume in the vertebra, showed significant subregional differences. Posterior margins of the vertebrae showed greater BMD than anterior regions, while central zones had the lowest BMD, lowest trabecular number and greatest trabecular separation. Generally vertebrae are less dense anteriorly and superiorly and more dense posteriorly and inferiorly with heterogeneity between symmetric sites within the vertebral body [2, 3, 25, 56, 67, 78]. The lower density and therefore lower compressive strength of the anterior zone of the vertebral body may account for the greater incidence of anterior crush fracture in individuals with osteoporosis. Therefore, a better understanding of the mechanics of vertebral fracture may be ascertained by investigating differences in subregional BMD between individuals with and without vertebral fracture. Overall vertebral BMD may remain the same in the presence of differential responses in trabecular architecture to mechanical loading [82]. The mechanisms underlying variation in subregional BMD remain unknown. However, it is likely that axial force transmission through the vertebral body may play a role. Adjacent musculature and the intervertebral disc modulate axial force.
3. Macroscopic factors
3.1. Vertebral Geometry
The shape of a vertebra is well designed to accept axial loads. The orientation and framework of the vertebral body assures that in normal circumstances, compressive load is transferred through the vertebral centrum, which by design, is the primary load bearing structure. This is highlighted by the observation that vertebral centrum size increases cranio-caudally as body weight percentage increases [9, 23, 87]. The vertebral body has a ratio of trabecular : cortical bone volume of 95:5, which explains its load bearing capacity when considering the function of the trabecular network [2]. Its design therefore provides the requirements for optimal load transfer by ensuring maximal strength with minimal weight [38].
Fracture risk is commonly based on measurements of vertebral BMD. However, other factors such as bone size and thickness of the cortical rim and endplates need to also be considered [16]. Consideration of these simple engineering principles may assist in fracture risk prediction [31]. Each of these factors is influenced independently with age, immobilisation, metabolic bone disease, disc degeneration and osteophyte formation. It is therefore difficult to determine the exact role of each factor and their individual contribution to the aetiology of vertebral fracture. The size of a vertebral body, often measured using computed tomography (CT) to calculate cross sectional area (CSA), has been found to be a predictor of risk of fracture [28, 65]. CSA provides an index of the ability to resist fracture given that bone strength is positively correlated with bone size [20].
Mechanical stress in a vertebral body during axial loading is inversely proportional to the CSA of the bone [16, 27]. Mazess et al. [54] found that individuals with osteoporosis had a significantly smaller vertebral CSA than individuals without osteoporosis. In a study comparing osteoporotic women with and without vertebral fracture, it was found that those with fracture had a reduced vertebral CSA in T12-L4 when matched for age, height, weight and BMD [28]. Another study comparing women with and without vertebral fracture found that vertebral volume was significantly lower in the fracture group when matched with controls for age, height, weight and BMD [15]. The smaller vertebral size in the fracture group would increase mechanical stress in the vertebral body, thereby limiting the loading capacity of the spine. Superimposing smaller vertebral size with the pathology of osteoporosis could increase fracture risk substantially.
4. Local Environment
4.1. Muscle Force
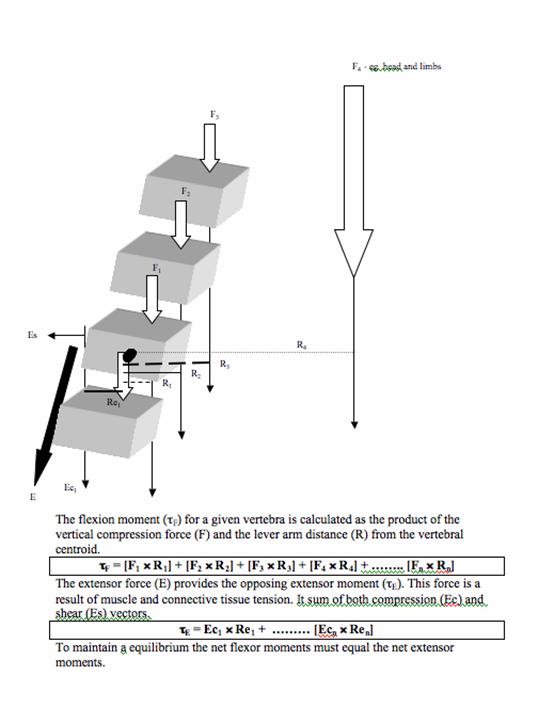
Muscles that attach to the vertebral column have a role in producing movement and providing protection to the spine by stabilising its structures. Without muscular support, the spine has a compression threshold of just 2kg before buckling [6, 66]. The paraspinal extensor muscles play an important role in maintaining static equilibrium of the trunk by resisting the flexion moment imposed by gravity and any mass carried anterior to the spine (see figure 2). The role of the erector spinae as a tonic muscle group has been confirmed by a histological study that found a predominance of type I muscle fibres (slow twitch, associated with endurance) at both thoracic and lumbar levels [50].
However, forces produced by muscle contraction may also be injurious to spinal structures. The muscles responsible for balancing spinal gravitational moments attach closely to the axis of rotation of the segment, therefore being at a mechanical disadvantage when considering the lever arm length. Relative to other muscles in the body, the spinal extensors have a very short lever arm. Therefore the force generated by these muscles must be sufficiently large to stabilise the trunk and overcome this mechanical disadvantage. The result is a higher compressive reaction force delivered to the intervertebral discs and vertebral centrum [14, 45, 68, 92]. Lever arm length can be affected by vertebral geometry and vertebral body position, as a function of spinal posture. Women have a smaller vertebral body size than men [6]. This helps to explain the observation that women have a 9% smaller lever arm from the erector spinae muscle to the centre of the vertebral body [27]. Gilsanz et al. [28] discovered that women with osteoporotic fractures had smaller lever arms (by 5.8%) than osteoporotic women with no fractures. The combination of reduced vertebral CSA and shorter lever arm resulted in increased mechanical loading by 8% during erect stance and by 15% during trunk flexion in the fracture cohort.
In a flexed spinal posture, the lever arm lengths of the lumbar erector spinae may decrease by up to 13.3% [9, 92]. Therefore, in order to maintain the same torque, muscle force must increase since torque is the product of force and lever length. Under normal conditions, the musculoskeletal system is able make this adjustment safely. However, given the pathology of osteoporosis, vertebral bone may be incapable of sustaining the resultant increase in compressive load. Vertebral fracture has been associated with an increase in thoracic kyphosis and a decrease in lumbar lordosis [84]. This change in spinal alignment will shorten the lever arm lengths since the spine will be positioned in greater flexion. Additionally, flexion will shift the body's line of gravity further anteriorly from the vertebral bodies. This will increase flexion moments, requiring large extensor counter-moments and therefore an increase in vertebral compression load. Compression force may also be increased due to the change in fibre orientation of the erector spinae when the spine flexes. Lumbar flexion is associated with a change in the line of action of longissimus and iliocostalis, thus reducing their capacity to resist shear force and increasing the compression vector [57]. Spinal posture and geometry may therefore significantly influence the risk of osteoporotic vertebral fracture.
Calculation of segmental vertebral load using a flexion moment at T5. The length of the lever arm (r) to the force vector (F) is partly related to the curvature of the spine at that level. The T12 lever arm is less than the T5 lever arm.
4.2. Muscle Strength
Spinal posture may be influenced by the strength of paraspinal muscles. Back extensor strength is negatively correlated with thoracic kyphosis in some studies [39, 84, 85], but not others [17]. The strength of muscles around the thoracic spine is believed to protect against thoracic fracture in patients with osteoporosis [85]. Several studies have shown back extensor strength decreases with age [17, 47, 83] and has an inverse relationship with BMD [74, 86, 83, 91], both of which are established correlates of osteoporosis. The magnitude of thoracic kyphosis is only moderately correlated with mean vertebral BMD and vertebral height ratios. This suggests that factors other than vertebral density and morphometry may contribute to kyphosis, for example muscle strength [19]. Extensor muscle weakness can result from an age-related decline in motor unit numbers, progressive habitual inactivity or pain inhibition [60]. As weakness in the extensor muscles increases, they are less able to maintain the necessary extensor moment to support an erect posture. Consequently kyphosis increases and the length of the extensor muscles increases. This lengthening may further reduce their torque generating capacity when considering the length-tension relationship of skeletal muscle [55]. As kyphosis increases, either as a result of muscle weakness or prevalent vertebral fracture, the compressive stress that vertebrae sustain increases as the flexion moment increases (see figure 2). This results in potential overload of the anterior portion of the vertebral body and structural failure. A mathematical modelling study by Keller et al. [42] found that an increase in kyphosis of 41.7º due to osteoporotic deformity resulted in a 25.2% reduction in spinal height and produced a 15.1cm anterior translation of C2. These changes resulted in a 19% increase in compressive force and a 40% increase in spinal extensor force required at T7/T8. Mueller et al. [64] found intramuscular pressure in the erector spinae to be significantly greater in individuals who adopted a kyphotic posture compared to an erect spinal posture, suggesting a significant increase in muscle tension with kyphosis. It is likely that as kyphosis increases, a vicious cycle of abnormal tissue loading will ensue, and if prolonged, will manifest as pathology, for example vertebral bone failure [30].
4.3. Neuromuscular control
In addition to the role of muscle activity in maintaining an erect spinal posture, the control of this activity should also be considered. The spine is inherently unstable. Muscles around the spine and trunk are responsible for providing controlled movement and stability. Maintaining controlled movement and stability in the trunk may minimise the risk of sustaining a vertebral fracture. To ensure stability and optimal movement control, the trunk muscles must have sufficient strength, endurance and correct recruitment patterns [71]. The patterns of recruitment and relative onset times between muscles are modulated by the central nervous system (CNS). Neuromuscular control relates to the role of the CNS in ensuring optimal muscle performance.
Prior research has confirmed the role of deep trunk muscles in maintaining stability of the lumbopelvic region [35, 36, 63]. Poor trunk stability as manifested through maladaptive muscle recruitment patterns and fatigue of deep abdominal (transversus abdominis) and deep spinal muscles (multifidus) may have an influential role in the aetiology of osteoporotic vertebral fracture. Research has confirmed that spinal intersegmental movement of 2º or more is associated with the development of local microtrauma [29]. Instability of the trunk may lead to a biomechanical overload of already weakened vertebrae and perpetuate subregional microtrauma due to suboptimal movement control and muscle recruitment.
Hodges and Richardson [36] suggested that dysfunction of the transversus abdominis (TrA) muscle would lead to suboptimal control of the spine against any forces that challenge its integrity. These researchers found that activation of TrA preceded other body movements sufficient to perturb the trunk in a normal population. A similar recruitment characteristic was noted for the deep fibres of multifidus [63]. When the same test was performed in subjects with low back pain, the TrA demonstrated latency in activation, suggesting dysfunction in neuromuscular control.
Other studies have found a decease in the CSA of multifidus in patients with chronic low back pain [11, 34, 33, 40]. Pain from symptomatic vertebral fracture may contribute to atrophy of the multifidus and further compromise intersegmental stability. Maladaptive motor control of the TrA and multifidus may also be related to other mechanisms such as alerted posture, muscle imbalance, pain and the development of a sedentary lifestyle. These factors may contribute to poor deep muscle control. O'Sullivan et al. [70] observed a decrease in multifidus activation in people who assumed a slumped sitting posture (thoracic and lumbar flexion). It is feasible that the increase in thoracic kyphosis, decrease in lumbar lordosis and change in sacral inclination associated with osteoporotic vertebral fracture may similarly affect paraspinal muscle control thereby compromising intersegmental spinal stability. This hypothesis is yet to be directly evaluated.
4.4. Intervertebral Disc Integrity
The intervertebral disc attaches to the vertebral body via the communication between the collagenous fibres of the annulus fibrosus and the vertebral end plate. The disc facilitates intersegmental movement while maintaining alignment of the vertebral column and has a primary role in attenuating and dispersing axial load. With aging, the water content of the nucleus pulposus decreases, compromising the properties of the disc and placing individuals at greater risk of vertebral injury [52]. As the disc degenerates, the mechanical load on the vertebral body may increase. Disc stiffness has been positively correlated to the degree of discal degeneration and amount of cyclical, repeated loading [41]. Any increase in disc stiffness will reduce movement at the motion segment. Therefore a potentially harmful increase in muscle force is required to obtain a desired movement. This response would obviously lead to greater mechanical stress through the vertebral body and thus increase the risk of fracture in an osteoporotic vertebral body.
The load bearing ratio between the cortex and trabecular bone depends on the integrity of the intervertebral disc. In healthy discs, the highest effective stresses are located in the centre of the bony end plates. For degenerated discs, the stresses are found in the lateral aspects of the end plates, in the cortical wall, and in the vertebral body rim [10, 37, 44]. These are areas of the vertebral body that have a lower threshold to axial load compared to intact trabecular bone in the centrum. Degenerative changes in the intervertebral disc have also been suggested to cause abnormal load distributions between the anterior and posterior zones of the vertebral body and neural arch [75-78]. These findings suggest that disc integrity can affect vertebral loading and potentially contribute to fracture risk by modulating changes in subregional BMD. Bone within the vertebral body could respond adaptively by altering density in response to changes in axial force transmission.
5. Global Environment
5.1. Body Position and Activity
The global environment relates to the activity and position of the body in space. As discussed previously, axial load transferred to the vertebral bodies is a function of body weight, tension in adjacent structures and intervertebral disc properties. Mechanical loading increases when body mass above the vertebra moves anterior to its axis of rotation. For example, loading will increase in situations where an individual bends forwards or rises from a chair. Duan et al. [16] estimated that activities associated with flexion of the upper body resulted in an approximate ten-fold increase in the compressive stress imposed on the vertebra compared to upright standing. For any loading scenario associated with flexion, the majority (92-100%) of the spinal stress has been attributed to the muscle-derived extensor moment and could therefore be considered as a significant contributor to the incidence of vertebral fracture [16]. The centre of mass will also move anterior to its axis of rotation with increasing kyphosis, which is a known correlate of osteoporotic vertebral fracture and back extensor muscle weakness. Furthermore, kyphosis is known to increase with successive vertebral fractures [4, 7, 8, 21].
Vertebral load will increase when compression forces increase, for example, carrying an additional load. The resulting compression force will then be a function of body weight, the mass of the load and the distance of the load to the vertebral body. Mannion et al. [51] found that for every increase in Newton weight held in the hands, there was a corresponding increase of compressive force of 5.23N on the lumbar spine. Vertebral fractures are assumed to occur from activities that maximise loads in vertebrae, particularly in vertebrae with altered extrinsic and intrinsic structural integrity. Peak loads in individuals will vary due to differences in activity levels and incidences of traumatic events such as falls [16]. Approximately 33% of vertebral fractures arise from falls however, in many cases the aetiology is unknown [5]. Osteoporotic vertebral fracture may simply be the result of weak vertebral bone being loaded during activities of daily living [42, 56, 59].
6. Conclusion and clinical implications
Physiologic load on vertebral bodies plays a significant role in the mechanics of vertebral fracture. However applying load estimates to predict fracture risk in vivo is difficult due to the complex geometry of the human skeleton. Loading estimates are often made with several assumptions, and this may threaten the internal validity of any model used. Furthermore, fracture risk prediction is complicated by the complexity in measuring skeletal loads during activities of daily living and traumatic events [31]. To achieve clinically sensitive measures of fracture risk, other factors should be considered in addition to our knowledge of spinal loading characteristics.
Bone microstructure, density, geometry and perhaps mineralisation will influence load bearing capacity. These factors are known to correlate well with the capacity of a vertebral body to withstand axial load, since both intrinsic (bone quality) and extrinsic (bone geometry) features can modulate load bearing capacity. Therefore, to arrive at more sensitive predictions of fracture risk, these factors should be considered and potentially measured simultaneously. Utilisation of QCT and high resolution magnetic resonance imaging (MRI) may present advantages over the more commonly used tools such as DXA and radiography. Estimations of multidimensional vertebral geometry cannot be made from DXA. Information derived from QCT allows isolation of densitometric and geometric changes in both cortical and trabecular bone [31]. MRI has the potential to make measurements of bone geometry and bone quality [22, 26, 48, 49].
Clinically, the advantages offered by QCT and MRI may be offset by the greater expense, longer scanning time and greater ionising radiation dosage delivered by QCT compared to DXA. With advances in DXA, lateral scanning of vertebral bodies in vivo with sufficient precision is now available. Clinical applications of DXA could be directed towards measuring areal BMD in vertebral subregions from lateral scans. However, the precision and accuracy of this application is yet to be evaluated. If these parameters were researched and optimised, clinically important information concerning fracture risk prediction and response to therapies may be provided.
The capacity of the osteoporotic spine to withstand axial loads may be influenced by trabecular bone quality, subregional BMD and vertebral body geometry. However, there is limited information in the literature regarding in vivo loading of the thoracic spine, other than a mathematical modelling study by Keller et al. [42]. It remains unclear if vertebral fractures significantly affect the segmental loading profile of the spine. Future research should be aimed at examining changes in loading profiles in individuals with osteoporotic vertebral fractures. Keller and colleagues [42] discuss the implication of increasing kyphosis on vertebral load, however it is not known if a magnitude threshold exists for kyphosis to significantly affect vertebral load and therefore fracture risk. To determine this, accurate and clinically applicable tools to measure thoracic kyphosis need to be appraised and applied to cohort studies. The effect of changes in spinal curvature and pain on the recruitment characteristics of thoracic paraspinal musculature in individuals with vertebral fractures also needs to be investigated. It is likely that changes in thoracic posture will influence the recruitment characteristics of these muscles as changes in strength, force vectors and length-tension relationships ensue.
With more information on subregional vertebral BMD, vertebral loading and neuromuscular control of trunk musculature, strategies to prevent further vertebral fracture and identification of individuals at high risk of fracture may be improved. Information about impairments in neuromuscular control and vertebral loading may assist health practitioners, such as physiotherapists, who treat patients with osteoporosis. Their treatment plans may be refined to include specific motor retraining and strengthening interventions and modalities to reduce vertebral loading. Additionally, specialists in bone health may be provided with valuable information on vertebral bone metabolism and the efficacy of pharmacotherapies by investigating subregional BMD within vertebral bodies.
Acknowledgements
The authors thank Mr Tim Wrigley and Professor Paul Hodges for their helpful comments.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Amling M, Posl M, Ritzel H. et al. Architecture and distribution of cancellous bone yield vertebral fracture clues: A histomorphometric analysis of the complete spinal column from 40 autopsy specimens. Arch. Orthop. Trauma Surg. 1996 ;115 :262 -269
2. Antonacci MD, Hanson DS, Leblanc A, Heggeness MH. Regional variation in vertebral bone density and trabecular architecture are influenced by osteoarthritic change and osteoporosis. Spine. 1997 ;22 (20) :2393 -2401
3. Banse X, Devogelaer JP, Munting E, Delloye C, Cornu O, Grynpas M. Inhomogeneity of human vertebral cancellous bone: Systematic density and structure patterns inside the vertebral body. Bone. 2001 ;28 (5) :563 -571
4. Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Falls prevention over 2 years: A randomized controlled trial in women 80 years and older. Age & Ageing. 1999 ;28 (6) :513 -518
5. Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ 3rd. Incidence of clinically diagnosed vertebral fractures: A population-based study in Rochester, Minnesota, 1985-1989. J. Bone Miner. Res. 1992 ;7 (2) :221 -227
6. Cooper RG, Hollis S, Jason MIV. Gender variation in human spinal and paraspinal structures. Clin. Biomech. 1992 ;7 :120 -124
7. Cortet B, Houvenagel E, Puisieux F, Roches E, Garnier P, Delcambre B. Spinal curvatures and quality of life in women with vertebral fractures secondary to osteoporosis. Spine. 1999 ;24 (18) :1921 -1925
8. Cortet B, Roches E, Logier R. et al. Evaluation of spinal curvatures after a recent osteoporotic vertebral fracture. Joint, Bone, Spine: Revue du Rhumatisme. 2002 ;69 (2) :201 -208
9. Daggfeldt K, Thorstensson A. The mechanics of back-extensor torque production about the lumbar spine. J. Biomech. 2003 ;36 (6) :815 -825
10. Dai L. The relationship between vertebral body deformity and disc degeneration in the lumbar spine of the senile. Eur. Spine J. 1998 ;7 :40 -44
11. Danneels LA, Vanderstraeten GG, Cambier DC, Witrouw EE, De Cuyper HJ. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur. Spine J. 2000 ;9 (4) :266 -272
12. De Smet AA, Robinson RG, Johnson BE, Lukert BP. Spinal compression fractures in osteoporotic women: Patterns and relationship to hyperkyphosis. Radiology. 1988 ;166 :497 -500
13. Diez F. Guidelines for the diagnosis of osteoporosis by densitometric methods. J. Manip. Physiol. Therap. 2002 ;25 (6) :403 -415
14. Dolan P, Adams MA. The Relationship between EMG activity and extensor moment generation in the erector spinae muscles during bending and lifting activities. J. Biomech. 1993 ;26 (4-5) :513 -522
15. Duan YB, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J. Bone Miner. Res. 1999 ;14 (10) :1796 -1802
16. Duan YB, Seeman E, Turner CH. The biomechanical basis of vertebral body fragility in men and women. J. Bone Miner. Res. 2001 ;16 (12) :2276 -2283
17. Eagan MS, Sedlock DA. Kyphosis in active and sedentary postmenopausal women. Med. Sci. Sport Exer. 2001 ;33 (5) :688 -695
18. Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ 3rd. Classification of vertebral fractures. J. Bone Miner. Res. 1991 ;6 (3) :207 -215
19. Edmondston SJ, Singer KP, Price RI, Day RE, Breidahl PD. The relationship between bone mineral density, vertebral body shape and spinal curvature in the elderly thoracolumbar spine: an in vitro study. Brit. J. of Radiol. 1994 ;67 :969 -975
20. Edmondston SJ, Singer KP, Day RE, Price RI, Breidahl PD. Ex vivo estimation of thoracolumbar vertebral body compressive strength: The relative contributions of bone densitometry and vertebral morphometry. Osteoporosis Int. 1997 ;7 (2) :142 -148
21. Ensrud KE, Black DM, Harris F, Ettinger B, Cummings SR. Correlates of kyphosis in older women. J. Am. Geriatr. Soc. 1997 ;45 (6) :682 -687
22. Faulkner KG, Pocock N. Future methods in the assessment of bone mass and structure. Best Pract. Res. Clin. Rheumatol. 2001 ;15 (3) :359 -383
23. Fazzalari NL, Manthey B, Parkinson IH. Intervertebral disc disorganisation and its relationship to age adjusted vertebral body morphometry and vertebral bone architecture. The Anatomical Record. 2001 ;262 :331 -339
24. Felsenburg D, Silman AJ, Lunt M. et al. Incidence of vertebral fracture in Europe: results from the European Prospective Osteoporosis Study (EPOS). J. Bone Miner. Res. 2002 ;17 (4) :716 -724
25. Flynn MJ, Cody DD. The assessment of vertebral bone microarchitecture with x-ray computed tomography. Calcif. Tissue Int. 1993 ;53 (Suppl 1) :S170 -S175
26. Genant HK, Gordon C, Jiang YB. et al. Advanced imaging of the macrostructure and microstructure of bone. Hormone Research. 2000 ;54 :24 -30
27. Gilsanz V, Boechat MI, Gilsanz R, Loro ML, Roe TF, Goodman WG. Gender differences in vertebral sizes in adults: Biomechanical implications. Radiology. 1994 ;190 :678 -682
28. Gilsanz V, Loro LM, Roe TF, Sayer J, Gilsanz R, Schulz EE. Vertebral size in elderly women with osteoporosis: Mechanical implications and relationships to fractures. The Journal of Clinical Investigation. 1995 ;95 (5) :2332 -2337
29. Gracovetsky S, Farfan H. The optimum spine. Spine. 1986 ;11 (6) :543 -573
30. Harrison DD, Harrison DE, Janik TJ, Cailliet R, Haas J. Do alterations in vertebral and disc dimensions affect an elliptical model of thoracic kyphosis? Spine. 2003 ;28 (5) :463 -469
31. Hayes WC, Piazza SJ, Zysset PK. Biomechanics of fracture risk prediction of the hip and spine by quantitative computed tomography. Radiol. Clin. N. Am. 1991 ;29 (1) :1 -18
32. Hayes WC, Myers ER. Biomechanical considerations of hip and spine fractures in osteoporotic bone. Inst. Course Lect. 1997 ;46 :431 -438
33. Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of Lumbar Multifidus Muscle Wasting Ipsilateral to Symptoms in Patients with Acute Subacute Low-Back-Pain. Spine. 1994 ;19 (2) :165 -172
34. Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine. 1996 ;21 (23) :2763 -2769
35. Hodges P, Cresswell A, Thorstensson A. Preparatory trunk motion accompanies rapid upper limb movement. Exp Brain Res. 1999 ;124 (1) :69 -79
36. Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain: a motor control evaluation of transversus abdominis. Spine. 1996 ;21 (22) :2640 -2650
37. Homminga J, Weinans H, Gowin W, Felsenberg D, Huiskes R. Osteoporosis changes the amount of vertebral trabecular bone at risk of fracture but not the vertebral load distribution. Spine. 2001 ;24 (14) :1555 -1561
38. Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000 ;405 (8) :704 -706
39. Itoi E, Sinaki M. Effect of back-strengthening exercise on posture in healthy women 49-65 years of age. Mayo Clin. Proc. 1994 ;69 :1054 -1059
40. Kader DF, Wardlaw D, Smith FW. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin. Radiol. 2000 ;55 (2) :145 -149
41. Kaigle A, Ekstrom L, Holm S, Rostedt M, Hansson T. In vivo dynamic stiffness of the porcine lumbar spine exposed to cyclic loading: Influence of load and degeneration. J. Spinal Disord. 1998 ;11 (1) :65 -70
42. Keller TS, Harrison DE, Colloca CJ, Harrison DD, Janik TJ. Prediction of osteoporotic spinal deformity. Spine. 2003 ;28 (5) :455 -462
43. Kopperdahl DL, Pearlman JL, Keaveny TM. Biomechanical consequences of an isolated overload on the human vertebral body. J. Orthop. Res. 2000 ;18 (5) :685 -690
44. Kurowski P, Kubo A. The relationship of degeneration of the intervertebral disc to mechanical loading conditions on lumbar vertebrae. Spine. 1986 ;11 (7) :726 -731
45. Lavender SA, Mirka GA, Schoenmarklin RW, Sommerich CM, Sudhakar LR, Marras WS. The effects of preview and task symmetry on trunk muscle response to sudden loading. Human Factors. 1989 ;31 (1) :101 -115
46. Legrand E, Chappard D, Pascaretti C. et al. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J. Bone Miner. Res. 2000 ;15 (1) :13 -19
47. Limburg PJ, Sinaki M, Rogers JW, Caskey PE, Pierskalla BK. A useful technique for measurement of back strength in osteoporotic and elderly patients. Mayo Clin. Proc. 1991 ;66 :39 -44
48. Link TM, Majumdar S, Grampp S. et al. Imaging of trabecular bone structure in osteoporosis. Eur. Radiol. 1999 ;9 (9) :1781 -1788
49. Majumdar S, Genant HK. A Review of the Recent Advances in Magnetic-Resonance-Imaging in the Assessment of Osteoporosis. Osteoporosis Int. 1995 ;5 (2) :79 -92
50. Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: Normal values and sex differences. J. Anat. 1997 ;190 :505 -513
51. Mannion AF, Adams MA, Dolan P. Sudden and unexpected loading generates high forces on the lumbar spine. Spine. 2000 ;25 (7) :842 -852
52. Martini FH. Fundamentals of Anatomy and Physiology, 3rd Edition. New Jersey: Prentice Hall. 1995
53. Mazess RB, Wahner HM. Nuclear medicine and densitometry. In: (ed.) Riggs BL, Melton LJ. Osteoporosis: Etiology, Diagnosis, and Management. New York: Raven Press. 1988 :251-295
54. Mazess RB, Barden H, Mautalen C, Vega E. Normalization of spine densitometry. J. Bone Miner. Res. 1994 ;9 (4) :541 -548
55. McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, nutrition, and human performance, 4 ed. Baltimore: Williams & Wilkins. 1996
56. McCubbery DA, Cody DD, Peterson EL, Kuhn JL, Flynn MJ, Goldstein SA. Static and Fatigue Failure Properties of Thoracic and Lumbar Vertebral Bodies and Their Relation to Regional Density. J. Biomech. 1995 ;28 (8) :891 -899
57. McGill SM, Hughson RL, Parks K. Changes in lumbar lordosis modify the role of the extensor muscles. Clin. Biomech. 2000 ;15 :777 -780
58. Melton LJ 3rd, Chao EYS, Lane JM. Biomechanical aspects of fractures. In: (ed.) Riggs BL, Melton LJ. Osetoporosis: Etiology, Diagnosis, and Management. New York: Raven Press. 1988 :111-131
59. Melton LJ 3rd, Kan SH, Frye MA, Wahner HW, O'Fallon WM, Riggs BL. Epidemiology of vertebral fractures in women. Am. J. of Epidemiol. 1989 ;129 (5) :1000 -1011
60. Miller RG. The effects of aging upon nerve and muscle function and their importance for neurorehabilitation. J. Neurol. Rehabil. 1995 ;9 (3) :175 -181
61. Mitra D, Elvins DM, Speden DJ, Collins AJ. The prevalence of vertebral fractures in mild ankylosing spondylitis and their relationship to bone mineral density. Rheumatology. 2000 ;39 (1) :85 -89
62. Mosekilde L. Vertebral bone quality and strength. In: (ed.) Cooper C, Reeve J. Spine: Vertebral Osteoporosis, vol 8. Philadelphia: Hanley & Belfus Inc. 1994 :63-81
63. Moseley GL, Hodges PW, Gandevia SC. Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine. 2002 ;27 (2) :E29 -36
64. Mueller G, Morlock MM, Volmer M, Honl M, Hille E, Schneider E. Intramuscular pressure in the erector spinae and intra-abdominal pressure related to posture and load. Spine. 1998 ;23 (23) :2580 -2590
65. Myers ER, Wilson SE. Biomechanics of osteoporosis and vertebral fracture. Spine. 1997 ;22 (24 Suppl) :25S -31S
66. Nachemson A. The load on lumbar discs in different positions of the body. Clin. Orthop. 1966 ;45 :107 -122
67. Nepper-Rasmussen HJ, Mosekilde L. Local differences in mineral content in vertebral trabecular bone measured by dual-energy computed tomography. Acta Radiologica. 1989 ;4 :369 -371
68. Nussbaum MA, Chaffin DB, Rechtien CJ. Muscle lines-of-action affect predicted forces in optimization-based spine muscle modelling. J. Biomech. 1995 ;28 (4) :401 -409
69. Oleksik A, Lips P, Dawson A. et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J. Bone Miner. Res. 2000 ;15 (7) :1384 -1392
70. O'Sullivan PB, Grahamslaw KM, Kendell M, Lapenskie SC, Moller NE, Richards KV. The effect of different standing and sitting postures on trunk muscle activity in a pain-free population. Spine. 2002 ;27 (11) :1238 -1244
71. Panjabi MM. The stabilizing system of the spine, Part I: Function, dysfunction, adaptation and enhancement. J. Spinal Disord. 1992 ;5 :383 -389
72. Parfitt AM. Bone remodelling: Relationship to the amount and structure of bone, and the pathogenesis and prevention of fracture. In: (ed.) Riggs BL, Melton LJ. Osteoporosis: Etiology, Diagnosis, and Management. New York: Raven Press. 1988 :45-93
73. Peel NFA, Moore DJ, Barrington NA, Bax DE, Eastell R. Risk of Vertebral Fracture and Relationship to Bone-Mineral Density in Steroid-Treated Rheumatoid-Arthritis. Ann. Rheum. Dis. 1995 ;54 (10) :801 -806
74. Pfeifer M, Begerow B, Minne HW. et al. Vitamin D status, trunk muscle strength, body sway, falls and fractures among 237 postmenopausal women with osteoporosis. Exp. Clin. Endocr. Diab. 2001 ;109 (2) :87 -92
75. Pollintine P, Dolan P, Tobias JH, Adams MA. Disc degeneration influences the distribution of load on the vertebral body: A cause of osteoporotic vertebral fractures in the elderly. J. Bone Miner. Res. 2002 ;17 (7) :OC6 -
76. Pollintine P, Farooq N, Park JC, Annesley-Williams DJ, Dolan P. Vertebroplasty can restore the mechanical function of the osteoporotic spine by relieving neural arch compressive loading. J. Bone Miner. Res. 2002 ;17 (7) :P43 -
77. Pollintine P, Garbutt SJ, Tobias JH. et al. Neural arch load-bearing can weaken the anterior vertebral body and pre-dispose to osteoporotic fracture. J. Bone Miner. Res. 2002 ;17 (7) :P41 -
78. Pollintine P, Tobias JH, McNally DS, Wakley GK, Dolan P, Adams MA. Intervertebral disc degeneration increases load-bearing by the neural arch and reduces BMD in the anterior vertebral body. J. Bone Miner. Res. 2002 ;17 :F9 -
79. Sandor T, Felsenberg D, Brown E. Comments on the hypotheses underlying fracture risk assessment in osteoporosis as proposed by the World Health Organization. Calcif. Tissue Int. 1999 ;64 :267 -270
80. Sievanen H, Kannus P, Nieminen V, Heinonen A, Oja P, Vuori I. Estimation of various mechanical characteristics of human bones using dual energy X-ray absorptiometry: Methodology and precision. Bone. 1996 ;18 (1) :S17 -S27
81. Silva MJ, Keaveny TM, Hayes WC. Load sharing between the shell and centrum in the lumbar vertebral body. Spine. 1997 ;22 (2) :140 -150
82. Simpson EK, Parkinson IH, Manthey B, Fazzalari NL. Intervertebral disc disorganisation is related to trabecular bone architecture in the lumbar spine. J. Bone Miner. Res. 2001 ;16 (4) :681 -687
83. Sinaki M, Khosla S, Limburg PJ, Rogers JW, Murtaugh PA. Muscle strength in osteoporotic versus normal women. Osteoporosis Int. 1993 ;3 :8 -12
84. Sinaki M, Itoi E, Rogers JW, Bergstralh EJ, Wahner HW. Correlation of back extensor strength with thoracic kyphosis and lumbar lordosis in estrogen-deficient women. Amer. J. Physical Med. 1996 ;75 (5) :370 -374
85. Sinaki M, Wollan PC, Scott RW, Gelczer RK. Can strong back extensors prevent vertebral fractures in women with osteoporosis? Mayo Clin. Proc. 1996 ;71 (10) :951 -956
86. Sinaki M, Fitzpatrick LA, Ritchie CK, Montesano A, Wahner HW. Site-specificity of bone mineral density and muscle strength in women: job-related and physical activity. Amer. J. Physical Med. 1998 ;77 (6) :470 -476
87. Singer K, Edmondston S, Day R, Breidahl P, Price R. Prediction of Thoracic and Lumbar Vertebral Body Compressive Strength - Correlations with Bone-Mineral Density and Vertebral Region. Bone. 1995 ;17 (2) :167 -174
88. Smit TH, Odgaard A, Schneider E. Structure and function of vertebral trabecular bone. Spine. 1997 ;22 (24) :2823 -2833
89. Spector TD, Hall GM, McCloskey EV, Kanis JA. Risk of Vertebral Fracture in Women with Rheumatoid-Arthritis. Brit. Med. J. 1993 ;306 (6877) :558 -
90. Spector TD, McCloskey EV, Doyle DV, Kanis JA. Prevalence of Vertebral Fracture in Women and the Relationship with Bone-Density and Symptoms - the Chingford Study. J. Bone Miner. Res. 1993 ;8 (7) :817 -822
91. Tsauo JY, Chien MY, Yang RS. Spinal performance and functional impairment in postmenopausal women with osteoporosis and osteopenia without vertebral fracture. Osteoporosis Int. 2002 ;13 :456 -460
92. Tveit P, Daggfeldt K, Hetland S, Thorstensson A. Erector Spinae Lever Arm Length Variations with Changes in Spinal Curvature. Spine. 1994 ;19 (2) :199 -204
93. World Health Organisation. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Report of a WHO study group. World Health Organisation Technical Report Series. 1994 :1-129
94. Yeh OC, Keaveny TM. Relative roles of microdamage and microfracture in the mechanical behavior of trabecular bone. J. Orthop. Res. 2001 ;19 (6) :1001 -1007
Author biography
Andrew M Briggs (BSc) is a PhD candidate at the School of Physiotherapy, University of Melbourne, Australia. He is investigating lumbar spine bone mineral density, thoracic vertebral loading and paraspinal muscle activity in individuals with osteoporosis.
Alison M Greig (BHk, BSc) is a PhD candidate at the School of Physiotherapy, University of Melbourne, Australia. She is investigating neuromuscular control characteristics of the trunk in individuals with osteoporosis.
John D Wark (PhD), Professor of Medicine, Endocrinologist, is Head of the Bone and Mineral Service at Royal Melbourne Hospital and Broadmeadows Osteoporosis Centre. He leads a team of researchers investigating bone health, quality and structure changes with related to genetics, exercise, maturation and pharmacotherapies.
Nicola L Fazzalari (PhD) is Associate Professor, Head of Bone and Joint Research Laboratory and Chief Medical Scientist (Division of Tissue Pathology) at the Institute of Medical and Veterinary Science, South Australia. Associate Professor Fazzalari is recognised for his studies of bone architecture and bone quality. His work is distinguished by its multidisciplinary approach to tissue analysis, using morphometric, molecular, biomechanical and mathematical analyses of human skeletal tissue.
Kim L Bennell (PhD) is Associate Professor and Director of the Centre for Health, Exercise and Sports Medicine, School of Physiotherapy, University of Melbourne, Australia. She leads a multidisciplinary team investigating musculoskeletal diseases across the lifespan.
![]() Corresponding address:
Corresponding address:
Andrew Briggs, Centre for Health, Exercise and Sports Medicine, School of Physiotherapy, University of Melbourne; Parkville, Victoria 3010, Australia. Tel: + 61 3 8344 4171 Fax: + 61 3 8344 4188 e-mail: a.briggs3unimelb.edu.au, k.bennelledu.au

 Global reach, higher impact
Global reach, higher impact