Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(3):152-164. doi:10.7150/ijms.1.152 This issue Cite
Review
An Increased Risk of Osteoporosis during Acquired Immunodeficiency Syndrome
Centre for Biotechnology, Acharya Nagarjuna University, Nagarjunanagar, Guntur- 522 510, A.P., India.
1Department of Venereology, GGH/SMC, NTR University Of Health Sciences, Vijayawada-520 002, A.P., India.
Received 2004-4-20; Accepted 2004-7-5; Published 2004-8-5
Abstract
Osteoporosis is characterized by decreased bone mineral density and mechanistic imbalances of bone tissue that may result in reduced skeletal strength and an enhanced susceptibility to fractures. Osteoporosis in its most common form affects the elderly (both sexes) and all racial groups of human beings. Multiple environmental risk factors like acquired immune deficiency syndrome (AIDS) are believed to be one of the causes of osteoporosis. Recently a high incidence of osteoporosis has been observed in human immunodeficiency virus (HIV) infected individuals. The etiology of this occurrence in HIV infections is controversial. This problem seems to be more frequent in patients receiving potent antiretroviral therapy. In AIDS, the main suggested risk factors for the development of osteoporosis are use of protease inhibitors, longer duration of HIV infection, lower body weight before antiretroviral therapy, high viral load. Variations in serum parameters like osteocalcin, c-telopeptide, levels of elements like Calcium, Magnesium, Phosphorus, concentration of vitamin-D metabolites, lactate levels, bicarbonate concentrations, amount of alkaline phosphatase are demonstrated in the course of development of osteoporosis. OPG/RANKL/RANK system is final mediator of bone remodeling. Bone mineral density (BMD) test is of added value to assess the risk of osteoporosis in patients infected with AIDS. The biochemical markers also aid in this assessment. Clinical management mostly follows the lines of treatment of osteoporosis and osteopenia.
Keywords: Osteoporosis, AIDS, HIV, Bone mineral density, HAART, Protease inhibitor, OPG/RANKL/RANK
1. INTRODUCTION
Osteoporosis is a significant cause of morbidity and mortality worldwide with an estimated ten million people in the United States already living with this disease [1]. It is a disease characterized by abnormalities in the amount and architectural arrangement of bone tissue, which lead to impaired skeletal strength and an increased susceptibility to fractures [2]. Bone is composed of organic component consisting of collagen which gives bone its flexibility, and a mineral component that includes calcium and phosphate salts, which combine to form hydroxyapatite crystals and add hardness to the collagen matrix. Osteoporotic condition manifests reduced bone mass with a loss of both collagen and minerals. Inspite of this loss, the collagen - calcium ratio is maintained at normal levels. However the reduced levels of both collagen and calcium compromise the bone strength leading to risk of fracture. Osteoporosis is characterized as either primary or secondary. Primary osteoporosis occurs in both sexes at all ages but often follows menopause in women and occurs later in life in men. In contrast secondary osteoporosis is a result of medication, other conditions, or diseases [3]. Many of the commonly occurring diseases like endocrine disorders, malabsorption disorders, bone marrow disorders and inflammatory diseases were reported to be associated with osteoporosis. In addition AIDS or HIV infection is also reported to play an important role in osteoporosis. In the recent days, high incidence of osteoporosis has been observed in HIV infected individuals [4-8].
AIDS is one of the known multiple environmental risk factors of osteoporosis [9]. HIV infection is associated with numerous metabolic and endocrine complications, leading to loss of appetite and hypogonadism. Medication during therapy also affect bone metabolism and contribute to bone loss [4,5]. Bone remodeling process is altered in HIV-infections which contributes to bone loss [6]. Weight loss, reduced lean body mass, and impaired functional capacity are the factors which may further predispose HIV infected individuals with wasting to bone loss. Reduced bone mineral density (BMD) has been demonstrated in HIV-infected men with hypogonadism [7] as well as in non-wasted HIV infected men [8]. Anabolic effects of testosterone and progressive resistance training therapy on lean body mass and muscle function in men with AIDS wasting have recently been reported [10,11]. Osteoporosis problem seems to be more frequent in patients receiving potent antiretroviral therapy, although a specific contribution of the drugs used in different combination regimens is yet to be established [12-15].
The introduction of highly active antiretroviral therapy (HAART) with the use of protease inhibitors (PI) has resulted in significant reductions in morbidity and mortality from HIV infection in recent years [16,17]. Practitioners have now become more cautious in early initiation of HAART in light of reports concerning serious and potentially irreversible toxicities associated with numerous antiretroviral drugs [18-24]. These toxicities include the development of diabetes mellitus, insulin resistance, hyperlipidemia, lipodystrophy and lactic acidosis [19-24]. The current review focuses on the possible factors that affect bone turnover and cause related bone disorders in AIDS.
2. EPIDEMIOLOGY OF OSTEOPOROSIS
A. MAGNITUDE OF THE PROBLEM
The number of people considered to have osteoporosis depends entirely on the way the condition is defined in practice. A committee of the World Health Organization (WHO) recommended the definitions shown in Table- 1 [25]. It is possible to diagnose and treat osteoporosis (bone density levels more than 2.5 standard deviations (SD) below the young normal mean) prior to the occurrence of fractures (established osteoporosis). This avoids the need to restrict treatment to end-stage disease, where it may be of limited effectiveness. It also circumvents the conceptual confusion that previously existed when a fracture occurred, without any change in underlying bone density [26]. Women with bone density levels between 1.0 and 2.5 SD below young normal mean levels have low bone mass, or osteopenia. Thus perimenopasual women with bone density that is 1 SD below the young normal mean might not have pathologically low bone mass but still they are at sufficiently high risk of fracture over their remaining lifetime that preventive therapy is indicted [27]. This is particularly important since currently available treatments can conserve existing bone mass but cannot restore osteoporotic bone to biomechanical normality [28].
World Health Organization Definition of Osteoporosis
| Bone Disorder | Bone Density in standard deviation (below the young adult mean) |
| Normal | >1 |
| Osteopenia | 1-2.5 |
| Osteoporosis | >2.5 |
| Severe Osteoporosis | >2.5 with fracture |
Source of table – Reference [25]
B. RISK FACTORS FOR OSTEOPOROSIS IN AIDS
The risk factors for osteoporosis are fairly straight forward (fig 1).
Schema of the object of environmental and genetic risk factors on the interaction between bone strength and trauma that leads to osteoporotic fracture. Source of Fig. - Reference [29]
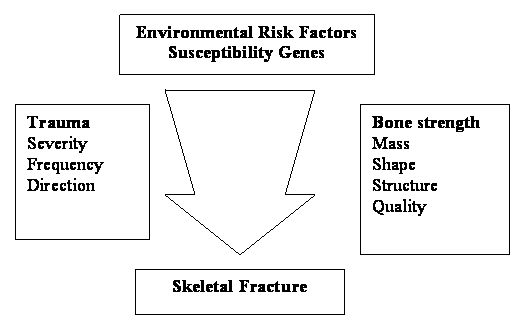
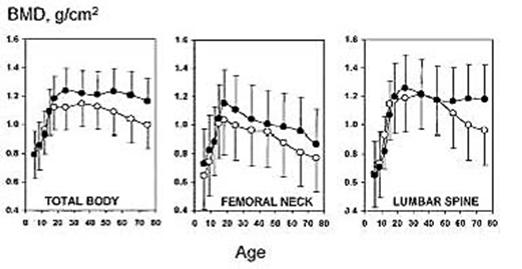
Osteoporosis in its most common form occurs in both the sexes of elderly and all racial groups affecting the BMD. The variations in bone mineral density in men and women are shown in fig. 2 [29]. A person's bone mass later in life is determined by the maximal bone mass achieved in young adulthood, as well as by the subsequent rate of bone loss. At the of age 70 years, these two determinants of bone mass are equally important [30]. The bone loss results from age-related factors that occur universally in the population and account for slow bone loss over life in both sexes. An accelerated phase of bone loss is associated with the menopause in women and hypogonadism in some men; and from medical and surgical conditions that produce secondary osteoporosis [28]. Thus race and sex differences in osteoporosis are explained in part by the heritability of skeletal size. Bone mass is greatest in those of African heritage, who have the lowest fracture rates, and is least in Caucasian women of Northern European origin, who have the highest fracture rates [32]. Similarly the accelerated phase of bone loss in perimenopasual women is superimposed upon the slower, age-related bone loss seen in both sexes and explains in part the two fold higher incidence of fractures among women than men later in life [28]. Other genetic risk factors play a major role in the heritability of many components of bone strength. There are a small number of cytogenetic [32,33] and monogenetic diseases causing osteoporosis [34-39]. Quantitative traits in bone strength in the normal population do not conform to a monogenetic mode of inheritance. The common form of osteoporosis is generally considered to be polygenic arising from the interactions of common polymorphic alleles at quantitative trait loci (QTL) with multiple environmental factors. Finding the genes underlying osteoporosis typically requires identification of its key heritable phenotypes and demonstrating in family and population studies that these phenotypes are coinherited with specific alleles. With progress in developing statistical methods to detect QTL and biochemical techniques to identify and map abundant polymorphisms throughout the genome studies [40,41] for identifying the susceptibile genes for osteoporosis made easy for timely study.
Microbial genome sequencing is likely to play an increasingly important role in the analysis of newly discovered human genes and provide further clues to the molecular basis of the diseases [42]. Because of the close correspondence among mammalian genomes, it is a hope that identification of the genes underlying bone strength in mammals such as the mouse [43] will be of major assistance for human studies. The identification of susceptibile genes for osteoporosis is expected to be a major contributing factor toward the long-term goal of understanding the molecular biology of the normal variation in bone strength and how it may be modified to prevent osteoporotic fractures. As with all genetic studies in humans, these scientific advances are need to be made in an environment of legal and ethical safeguards that are acceptable to the general public [44].
Normal variation (mean and 2 8D) and change in BMD with age in healthy men (black circle) and women in (open circle) (Normal population Data base, DPX-IQ Reference Manual, Documentation Version 5/96, Lunar Corp., Maidson, WI). Peak bone mass at hip and spine for measurement on Lunar machines is taken as the mean BMD between age 20 and 40 yr, but this age range varies with DXA machine manufacture. Source of Fig. - Reference [29]
In AIDS, the mainly suggested risk factors for the development of osteopoenia and osteoporosis are the use of protease inhibitors, longer duration of HIV infection, high viral load, high lactate levels, low bicarbonate levels, raised alkaline phosphatase level, and lower body weight before antiretroviral therapy. There have also been a few case reports of pathologic fractures in AIDS patients with antiretroviral therapy-induced osteopenia and osteoporosis. The underlying mechanism triggering bone loss in HIV infected patients is still unknown [3].
3. BONE TURNOVER
A. BONE REMODELING
Growth in bone size and strength occurs during childhood, but bone accumulation is not completed until the cessation of linear growth (till the third decade of life). Even after bone accumulation has ended, there is a constant state of remodeling with repeated cycles of resorption followed by the deposition of new bone [3].
The cellular process of bone activity by which both cortical and cancellous bone is maintained is referred to as bone remodeling. This bone remodeling process takes place in discrete packets known as multicellular units [45]. Initially, a cell from the hematopoietic granulocyte-macrophage colony-forming unit lineage (along a bone surface) is activated and proliferates or transforms into an osteoclast [46]. An osteoclast creates bone cavity and subsequently, osteoblasts, derived from pleuripotent mesenchymal stem cells of the bone marrow, fill in the area of resorption with type 1 collagen [47]. Type I collagen ultimately becomes mineralized, probably as a function of the osteoblasts, thereby completing the process of new bone formation. There is an interdependency of the osteoclastic and osteoblastic activities where by osteoclasts are initially recruited to a particular site on the bone surface, and when their task is completed, they signal the osteoblasts to attend to that same site. This interrelationship is known as coupling and is crucial link in the chain of bone-remodelling events [48]. A situation that interferes with coupling process or that causes imbalances between bone forming and resorbing relationship leads to significant loss of bone over the time.
Regulation of the bone-remodeling process is complex. Undoubtedly, there are numerous systemic hormones, such as parathyroid hormone, 1, 2 5-dihydroxy-vitamin D (calcitriol), calcitonin, estrogens, and androgens that regulate the process. Vitamin D is recognized as a stimulator of osteoclastic formation and a promoter of osteoblast differentiation [49]. There are also numerous local factors that play an important role in the physiology of bone remodeling like interleukins (IL-1 and IL-6), transforming growth factors (TGF), prostaglandins, tumor necrosis factor (TNF), lymphotoxin, colony stimulating factors (CSF), and gamma interferons [50-53].
OPG/RANKL/RANK system is the dominant, final mediator of osteoclastogenesis. This system explains the precise mechanisms by which preosteoblastic stromal cells control the osteoclast development. It is a specific factor produced by preosteoblastic/stromal cells that is both necessary and sufficient for osteoclast development. Osteoprotegerin (OPG) is a secreted soluble member of the tumor necrosis factor receptor superfamily (TNFR), also known as osteoclastogenesis inhibitory factor (OCIF) [54,55]. It has specificity for OPG/OCIF function for inhibiting osteoclast differentiation. The initial cloning and characterization of OPG as a soluble, decoy receptor belonging to the TNF receptor superfamily is the first step that eventually led to an unraveling of this system. Soon thereafter, the molecule blocked by OPG, initially called OPG-ligand/osteoclast differentiating factor (ODF). RANKL, is the key mediator of osteoclastogenesis in both a membrane-bound form expressed on preosteoblastic/stromal cells as well as a soluble form. RANKL, in turn, binds to its receptor, RANK, on osteoclast lineage cells. The decisive role played by these factors in regulating bone metabolism was demonstrated by the findings of extremes of skeletal phenotypes (osteoporosis vs. osteopetrosis) in mice with altered expression of these molecules. Identifying the factors regulating this system, the signaling mechanisms involved in the RANKL/ RANK pathway, and finally, potential alterations in this system in metabolic bone disorders that develop during HIV infections are crucial in understanding the mechanism underlying osteoclastogenesis in this particular type [56].
B. INTERACTIONS BETWEEN SKELETAL , IMMUNE AND HORMONAL SYSTEMS
The entry of HIV-1 into target cells requires the binding of the viral envelope glycoprotein (Env) with the target cell CD4 and an additional target cell co-receptor [57]. The co-receptors required for the fusion of the T cell-tropic and macrophage-tropic viruses with their target cells have now been identified to be fusin and CCR-5, respectively [58,59]. Fusin is a 7 TM domain protein with significant amino acid sequence homology to the IL-8 receptors. A CXC chemokine, PBSF/SDF-1, has recently been identified to be a ligand for fusin [60,61] The identification of CCR-5 as the co-receptor for macrophage-tropic viruses is consistent with an earlier report identifying the CCR-5 ligands (RANTES, MIP-1α and MIP-1β) as the major HIV suppressive factors produced by CD8+ T cells for macrophage-tropic, but not T cell tropic, HIV isolates [62]. Besides fusin and CCR-5, use of other chemokine receptors such as CCR-2B and CCR-3 by a minority of HIV isolates has also been reported [63].
The hypothesis that the systemic activation of T cells in vivo leads to an osteoprotegerin ligand-mediated increase in osteoclastogenesis and bone loss [64]. This explains the interaction of HIV infection and bone mineralization. When the homeostasis between RANK/RANKL- osteoprotegerin is lost, there is an increased incidence of loss of bone mineral density. Interactions between cells of skeletal and immune system are important for the maintenance of bone homeostasis [65]. These cellular circuits are in part mediated by specific cytokines and changes in levels of these mediations may result in altered bone remodeling and disease [65-67]. Cytokines such as IL-1, IL-6, IL-11 and TNF-α may stimulate osteoclast activity [66-68] and enhanced IL-6 levels appear to play a pathogenic role in the enhanced bone resorption of post menopasual osteoporosis [69]. Some of these cytokines may also inhibit bone formation exerted by their negative regulatory effects on osteoblasts [65, 70,71]. This inhibitory effect on osteoblats combined with stimulation of osteoclasts suggest a pathogenic role for these cytokines in bone disorders characterized by increased resorption combined with decreased formation of bone [65].
Persistent activation of proinflamatory cytokines such as IL-1 and in particular TNF- α appears to play an important pathogenic role in HIV [72-75]. This proinflamatory activation may enhance HIV replication, contributing to the development of immunodeficiency and certain clinical manifestations [74,75] and also be related to the endocrine abnormalities seen in HIV infected individuals [76,77]. Vitamin D metabolites and elements like Calcium, Magnesium, Phosphorus have complex effects in the bone system, with stimulatory effects on both formation and resorption [65,78].
Estrogen deficiency causes an increase in osteoclastic resorbing capacity [79-81]. Estrogen deficiency both directly and indirectly decreases the efficiency of intestinal and renal calcium absorption and reabsorption respectively. Testosterone deficiency in men is the major identifiable cause of male osteoporosis. It has analogous mineral metabolism effects. Evidence in support of gonadal hormone deficiency as the cause of increasing bone resorption is that specific receptors for estrogen and testosterone have been identified on the surface of the bone cells [82].
4. BONE MINERAL DENSITY IN HIV INFECTIONS
Bone mineral density is widely accepted as a measure of bone strength. BMD measurements have been shown to correlate strongly with the load bearing capacity of the hip and spine and with the risk of fracture. In patients with osteoporosis, there is a four fold to five fold increase in risk for fracture. And for patients with osteoporosis and a history of fracture, the risk of another fracture occurring is increased twenty fold.
While decreased BMD is certainly an important prediction of fracture risk, it is not the only parameter to consider. Fracture risk is also associated with a history of falls, low physical function such as slow gait speed and decreased quadriceps, impaired cognition and vision, and the presence of environmental hazards [3].
A. BMD in HIV infected individuals prior to use of Highly Active Anti Retroviral Therapy (HAART)
Prior to the widespread use of HAART, studies indicated that bone metabolism was altered, albeit minimally, in HIV infected individuals. Before the availability of protease inhibitors (PIs), low BMD was rarely observed in HIV infected individuals. However, the role of HAART in the reduction of BMD is controversially reported. According to some reports the direct correlation between the use of PI and osteoporosis is not so evident. BMD was significantly lower in HIV-seropositive patients in comparison with controls in lumbar spine, proximal femur and total body, without significant differences among treatment-naive patients and either of the treatment groups. Only time with HIV infection and not specific therapy was associated with BMD decreases [83] patients not receiving antiretrovirals also have a higher than expected prevalence of reduced BMD, which suggests that HIV itself may be a contributing factor, mediated by immune activation and cytokines [84]
In one analysis, 45 HIV-infected patients had statistically significant lower lumbar spine BMD scores than did HIV-negative controls. The subjects and controls did not differ in total or hip BMD. A small decrease in total body BMD was observed in a longitudinal follow up after 15 months. No significant reduction was found in spine and hip BMD. This matched the lines of osteopenia rather than osteoporosis [85]. But advanced stages of HIV infection demonstrated lower BMD of the individuals than HIV-negative controls. Several additional studies suggest a significant prevalence of low BMD in HAART-naive patients. This shows the probable role of HIV infection itself in decreasing BMD. The prevalence of osteoporosis in HAART-naive population is found to be approximately 28%, compared with the expected 16% in the general population [86-88]
Studies associated with biochemical markers of bone metabolism and bone biopsy of therapy-naïve HIV-infected individuals show a significant decrease in osteocalcin, a marker of bone formation and a marked increase in C-telopeptide, a marker of bone resorption in a comparison with healthy HIV-seronegative controls. These facts correlate with enhanced activation of the tumor necrosis factor system and increasing severity of HIV disease [89-91]. Numerous cytokines are known to induce differentiation of bone marrow precursors into osteoclasts. Hence bone resorption and osteoporosis are probably favoured. Abnormal immune system activation may be one of the probable causes that lead to bone resorption.
A study of bone samples from anti-retroviral therapy-naïve HIV infected subjects compared with healthy controls showed only decreased levels of osteocalcin in individuals with lower CD4 counts. No alterations in BMD or biochemical differences in bone metabolism were demonstrated [92].
B. BMD in HIV infected individuals after the use of HAART
A cross-sectional analysis of whole body, lumbar spine and proximal femur BMD in male subjects receiving HAART that included a protease inhibitor (PI), HIV-infected patients not receiving a PI and healthy seronegative adults using dual x-ray absorptiometry (DXA) scans was performed. Men receiving PI had lower lumbar spine BMD compared with the other two groups. 50% of the subjects on PIs were classified as osteopenic or osteoporotic according to WHO classification [13]. An assessment of bone metabolism in HIV infected subjects receiving HAART with 2 nucleosides and a PI [93] showed 43% of the subjects to be osteopenic or osteoporotic according to the WHO definition. Increased markers of bone resorption and bone formation, including elevations in urine pyridinolines, bone alkaline phosphatase, and osteocalcin. Another study in HIV-infected children also demonstrated HAART-associated losses in BMD that were associated with an increased rate of bone turnover [94]. Other studies have shown a more accelerated loss of BMD in individuals receiving potent antiretroviral therapy, but the association with PI use remains speculative and needs to be confirmed [14-15].
It is impossible to attribute cause and effect and to measure the cumulative effects of other important but common risk factors for BMD loss in HIV-infected individuals. Prospective and longitudinal studies are necessary to determine the exact nature and mechanism of each individual factor in the pathogenesis of HIV-related bone mineral loss.
5. ASSESSMENT OF BONE REMODELING
Bone remodeling can be assessed using surrogate markers of bone turnover in the blood or urine. These markers include bone-specific alkaline phosphatase and osteocalcin, which are indices of bone formation. There are also urinary levels of pyridinolines and deoxypyridinolines and serum and urine levels of type 1 collagen telopeptides (CTX and NTX), which are indices of bone resorption. The level of these markers may identify changes in bone remodeling within a relatively short time - several days to months - before changes in bone density can be detected. It is important to realize, however, that marker levels cannot predict fracture risk and are only weakly associated with changes in bone mass. Therefore, they are of limited utility in the clinical evaluation of individual parametres [3].
6. CLINICAL MANAGEMENT
Current diagnosis and treatment of osteoporosis is primarily based on recommendations used for the management of disease in HIV-seronegative adults. Measurement of BMD as a routine test in HIV-infected patients is not recommended. However, plain radiographs and magnetic resonance imaging are the cornerstones of diagnosis. A detailed history of the past, physical examination, evaluation of nutritional status and potential secondary causes of osteoporosis are to be taken into consideration before the onset of the treatment of HIV-infected patients. Abnormal laboratory values of elevated alkaline phosphatase or low testosterone obtained during the course of HIV treatment should not be ignored during treatment [94]. The primary diagnosis of osteoporosis is based on the WHO definitions according to measurements of BMD. The most widely used technique for determining BMD is DXA. DXA scanning measures bone mass and density in central regions of interest (hip and spine) as well as appendicular regions (wrist, forearm, heel). It has become the gold standard to which all other bone densitometry techniques are compared [95-97] Techniques for determining bone mineral density at various sites are summarized in Table 2. DXA studies are also useful to define fracture risk as well as to measure the efectiveness of various therapies on bone mass. Biochemical markers of bone turnover can provide complemetnary information to DXA scans, including changes in bone remodeling that can be identified before changes in BMD [1]. However the exact effectiveness of such markers remains controversial.
Techniques for determining bone mineral density at various sites
| Technique | Sites |
| Central dual-energy x-ray absorptiometry | Spine, hip, whole body |
| Peripheral dual-energy x-ray absorptiometry | Middle finger, wrist, heel, forearm |
| Single-energy x-ray absorptiometry | Forearm, heel |
| Quantitative computed tomography | Trabecular bone only; vertebral body |
| Peripheral quantitative computed tomography | Forearm |
| Ultrasound | Heel, patella, ankle |
Management is dependent on the stage of bone disease and ranges from observation to total joint arthroplasty. Clinicians may help to prevent HIV-associated osteonecrosis by encouraging patients to limit their exposure to the established risk factors for the disease [98]. The treatment strategies that are effective in the general population should be pursued. Reversible causes of secondary osteoporosis should be studied. Vitamin D and calcium intake should be optimized. Other nutrients are also important in relation to bone health. Diets with very high protein content, excess caffeine, phosphorus, and sodium can increase calcium losses. Moderate physical activity is also recommended. The treatment for such conditions usually follows the general lines of management of osteoporosis [1].
Patients undergoing long-term corticosteroid treatment should begin primary prevention measures as soon as such agents are prescribed. Attempts to preserve bone should not be delayed until the underlying disease process is under way. Any patient taking glucocorticoids who has a T score of less than -1.0 should immediately be given pharmacologic treatment [99]. The most commonly used pharmacologic treatments for osteoporosis (excluding calcium and vitamin D supplements) are antiresorptive agents (estrogen, bisphosphonates, calcitonins, and selective estrogen receptor modulators). Other agents under development or already in use outside the United States include fluoride salts, parathyroid hormone, active forms of vitamin D (calcitriol, alfacalcidol), and anabolic steroids.
HRT
The utility of HRT (estrogen) for prevention of bone loss in early menopause is well established. HRT that is started at menopause retards or prevents bone loss and increases BMD somewhat. HRT continues to prevent bone loss for as long as it is taken, but bone loss resumes when estrogen is discontinued [100,101]. HRT is also effective in older women with established osteoporosis. Added potential benefits of HRT include controlling menopausal symptoms and reducing the risk of heart disease. Despite its documented benefits, however, some women find that the side-effect profile of HRT (eg, breast tenderness, abnormal uterine bleeding, endometrial hyperplasia, migraine, deep venous thrombosis) is unacceptable. Additionally, women may fear the relationship between HRT and breast cancer. Nevertheless, hormone replacement is considered first-line therapy in most postmenopausal patients.
Bisphosphonates
Candidates for bisphosphonate treatment include premenopausal women at increased risk for osteoporosis, postmenopausal women who forgo HRT, men with osteoporosis [102,103], and all individuals receiving high-dose corticosteroid therapy. In controlled clinical trials [104,105], bisphosphonates reduced the risk of fractures of the spine, hip, and wrist by 40% to 50% in postmenopausal women. These data are particularly significant because no randomized clinical trials have actually measured the effect of HRT on hip fracture, the most serious consequence of osteoporosis, even though observed studies consistently show that postmenopausal women who have been receiving HRT for 5 to 10 years have a lower risk of hip fracture than their counterparts who have not [106]
Several studies have shown bisphosphonates to be highly effective for prevention of glucocorticoid-induced bone loss, and these drugs have been approved by the US Food and Drug
Administration (FDA) for this indication. Risedronate sodium (Actonel), a pyridinyl bisphosphonate with FDA approval for the treatment of Paget's disease of bone, has recently been approved for prevention and treatment of postmenopausal and glucocorticoid-induced osteoporosis. Recent data from two controlled studies showed that this agent reduced the incidence of vertebral fractures by 70% in patients beginning corticosteroid therapy (when bone loss is most rapid) compared with controls [107].
Clinical experience with bisphosphonates has shown that patients may experience upper gastrointestinal disturbance, particularly esophageal symptoms (heartburn, painful or difficult swallowing) .Alendronate sodium (Fosamax) should be taken with 6 to 8 oz of plain water at least one-half hour before the first food, beverage, or medication of the day. Other beverages (including mineral water), food, and other medications can reduce the absorption of oral bisphosphonates. Also, patients should not lie down until at least 30 minutes after taking alendronate and until after the first food of the day [108]. Clinical trials of risedronate have included postmenopausal women with ongoing gastrointestinal disease and those using aspirin or nonsteroidal anti-inflammatory drugs. Preliminary results [109] indicate that the incidence and severity of gastrointestinal events with use of risedronate are similar to those reported in the control group. Alternative dosage formulation and administration of bisphosphonates is under investigation.
Calcitonins
For patients who are unable or choose not to undergo HRT or take a bisphosphonate, a calcitonin is a viable alternative. Calcitonin-salmon is administered as a nasal spray (Miacalcin) or by injection. Calcitonin prevents bone loss and fracture in established osteoporosis, although it is somewhat less effective than HRT and bisphosphonates [110]. Recent results of a large controlled study (Prevent Recurrence of Osteoporotic Fracture) showed that calcitonin-salmon nasal spray reduced the incidence of new spinal fractures by 36% over a 5-year period, compared with placebo, in postmenopausal women who had previously experienced fracture. To date, calcitonin has shown no effect on nonvertebral fractures, and it has not been shown to reduce fracture risk in corticosteroid-treated patients [111]. However, calcitonin has modest analgesic properties, which may decrease opioid use and allow earlier ambulation in patients with acute vertebral fractures.
Selective estrogen receptor modulators
A selective estrogen receptor modulator may serve as an alternative to HRT for selected patients. Raloxifene (Evista) is the most studied of these drugs to date; its estrogenlike effects increase BMD . Raloxifene decreases total and low-density lipoprotein cholesterol levels, but unlike HRT, it does not affect high-density lipoprotein cholesterol. Also, unlike HRT, raloxifene does not appear to stimulate the endometrium . Recent data from the multiple outcomes of Raloxifene evaluation showed that 60- and 120-mg daily doses of raloxifene significantly decreased vertebral fracture risk during the first 36 months of treatment, compared with placebo. All patients also received calcium and cholecalciferol. At 36 months, raloxifene did not significantly lower the risk of nonvertebral fractures compared with placebo, although the cumulative incidence of nonvertebral fractures in active and placebo groups begins to diverge at 2 years. The effect of raloxifene on hip fracture is under investigation [112,113]. Specific studies with HIV-infected individuals should be conducted to determine the most effective way of this treatment.
7. CONCLUSION
Osteoporosis has been the recently recognized complication affecting HIV-positive patients. Etiology and pathogenesis of osteoporosis in HIV infection are still uncertain. Progression in HIV infection and HAART with PI are the possible factors that precipitate osteoporosis in HIV–sero-positive patients. Further studies should be extended to look into the natural history of bone loss during HIV disease. This would help to understand the mechanisms of uncoupled bone turnover and effects of PI therapy on HIV infected patients. As the number and life expectancy of HIV-positive patients treated with HAART increases, the development and treatment of osteoporosis in HIV infection should be taken into consideration in the long term management of HIV disease.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. NIH. NIH Consensus Development Panel on Osteoporosis, Prevention, Diagnosis, and Therapy. JAMA. 2001 ;285 :785 -95
2. Peck WA. et al. Consensus Development Conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993 ;94 :646 -650
3. Tebas P. Osteopenia, Osteoporosis, and Other Bone Problems in HIV-Infected individuals. The PRN Notebook. 2001 :- 6(3)
4. Grinspoon SK. et al. HIV Disease and the endocrine system. N Engl J Med. 1992 ;327 :1369 -1365
5. Corcoran C. et al. Treatments for wasting in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1999 ;340 :1740 -1750
6. Aukurst P. et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999 ;84 :145 -150
7. Stephens E. et al. Symptomatic osteoporosis in two young HIV-positive African women. AIDS. 1999 ;13 :2605 -2606
8. Paton NIJ. et al. Bone mineral density in patients with human immunodeficiency virus infection. Calcif Tissue Int. 1997 ;61 :30 -32
9. Fairfield WP. et al. Osteopenia in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J Clin Endocrinol Metab. 2001 ;86 :2020 -
10. Bhasin S. et al. Testosterone replacement and resistance exercise in HIV-infected men with weight loss and low testosterone levels. JAMA. 2000 ;283 :763 -770
11. Grinspoon S. et al. Effects of testosterone and progressive resistance training in eugonadal men with AIDS wasting, A randomized, controlled trial. Ann Intern Med. 2000 ;133 :348 -355
12. Meyer D. et al. Osteonecrosis of the femoral head in patients receiving HIV protease inhibitors. AIDS. 1999 ;13 :1147 -8
13. Tebas P. et al. Accelerated bone mineral loss in HIV infected patients receiving potent antiretroviral therapy. AIDS. 2000 ;14 :63 -7
14. Hoy J. et al. Osteopenia in a randomized, multicenter study of protease inhibitor (PI) substitution in patients with the lipodystrophy syndrome and dwell controlled HIV viremal [abstract 208]. Program and abstracts of the 7th Conference on Retroviruses and opportunistic infections. Washington, DC: Foundation for Retroviruses and Human Health. 2000 :114-
15. Negredo E. et al. Bone mineral density (BMD) in HIV-1-infected patients [abstract 626]. Program and abstracts of the 8th Conference on Retroviruses and Opportunistic Infections. Washington, DC: Foundation for Retroviruses and Human Health. 2001 :232-
16. Hammer SM. et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 counts of 200 per cubic millimeter or less: AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997 ;337 :725 -33
17. Palella FJ. et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection: HIV Outpatient Study Investigators. N Engl J Med. 1998 ;338 :853 -60
18. Brinkman K. et al. Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as a common pathway. AIDS. 1998 ;12 :1735 -44
19. Carr A. et al. A syndrome of peripheral lipodystrophy hyperlipidemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998 ;12 :F51 -8
20. Miller KD. et al. Lactic acidosis and hepatic steatosis associated with use of stavudine: report of four cases. Ann Intern Med. 2000 ;133 :192 -6
21. Chariot P. et al. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J Hepatol. 1999 ;30 :156 -60
22. Behrens G. et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999 ;13 :F63 -70
23. Miller KD. et al. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet. 1998 ;351 :871 -5
24. Flynn TE. et al. Myocardial infarction in HIV-infected men receiving protease inhibitors. Ann intern Med. 1999 ;131 :548 -
25. Kanis JA. et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994 ;8 :1137 -1141
26. Schapira D, Schapira C. Osteoporosis: the evolution of a scientific term. Osteoporosis Int. 1992 ;2 :164 -167
27. Johnston CC Jr. et al. Clinical indications for bone mass measurements: a report from the Scientific Advisory Board of the National Osteoporosis Foundation. J Bone Miner Res. 1989 ;4 (suppl 2) :1 -28
28. Riggs BL. et al. The prevention and treatment of osteoporosis. N Engl J Med. 1992 ;327 :620 -627
29. Riggs BL. Osteoporosis. In: (ed.) Wyngaarden JB, Smith LH Jr. Cecil's Textbook of Medicine. Philadelphia: WB Saunders Company. 1988 :1510-1515
30. Hui SL. et al. The contribution of bone loss to post menopausal osteoporosis. Osteoporosis Int. 1990 ;1 :30 -34
31. Melton LJ III. Differing patterns of osteoporosis across the world. In: (ed.) Chesnut CH III. Proceedings of the Second Asian Symposium on Osteoporosis- New Dimensions in Osteoporosis in the 1990s. Hong Kong: Excerpta Medica Asia 1991 13-18
32. Bertelloni S. et al. Volumetric bone mineral density in young women with Turner's syndrome treated with estrogens or estrogens plus growth hormone. Horm Res. 2000 ;53 :72 -76
33. Horowitz M. et al. Osteoporosis and Klinefelter's syndrome. Clin Endocrinol (Oxf). 1992 ;36 :113 -118
34. Prockop DJ. et al. Mutations in type 1 procollagen that cause osteogenesis imperfecta: effects of the mutations on the assembly of collagen into fibrils, the basis of phenotypic variations, and potential antisense therapies. J Bone Miner Res. 1993 ;8 (Suppl 2) :489 -492
35. Lubec B. et al. Evidence for McKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta Mol Basis Dis. 1996 ;1315 :159 -162
36. Goto M. Hierarchical deterioration of body systems in Werner's syndrome. Mech Ageing Dev. 1997 ;98 :239 -
37. Smith EP. et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994 ;331 :1056 -1061
38. Carani C. et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997 ;337 :91 -95
39. Gong Y. et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001 ;107 :513 -523
40. Lander ES. et al. Genetic dissection of complex traits. Science. 1994 ;265 :2037 -2048
41. Weeks DE. et al. Polygenic disease: methods for mapping complex disease traits. Trends Genet. 1995 ;11 :513 -519
42. Sushma Deepthi A. et al. Leishmaniasis: Progress in Vaccine Development. Pharma Bio World. 2004 ;2 :75 -78
43. DeBry RW. et al. Human/mouse homology relation-ships. Genomics. 1996 ;33 :337 -351
44. Meslin EM. et al. The ethical, legal and social implications research program at the National Human Genome Research Institute. Kennedy Inst Ethics J. 1997 ;7 :291 -8
45. Parfitt AM. Bone remodeling and bone loss: understanding the pathophysiology of osteoporosis. Clin Obstet Gynecol. 1987 ;30 (4) :789 -811
46. Christiansen C. Skeletal osteoporosis. J Bone Miner Res. 1993 ;8 (2) :S475 -S480
47. Puzas JE. The osteoblast. In: (ed.) Favus MJ. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 2nd ed. New York: Raven Press. 1993 :15-21
48. Parfitt AM. Morphologic basis of bone mineral measurements: transient and steady state effects of treatment in osteoporosis. Min Electr Metab. 1980 ;14 :273 -287
49. DeLuca HF. Vitamin D revisited. Clin Endocrinol Metab. 1980 ;9 :1 -26
50. Vaananen HK. Pathogenesis of osteoporosis. Calcif Tissue Int. 1991 ;49 (suppl) :S11 -S14
51. Epstein FH. Bone marrow, cytokines, and bone remodeling: emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995 ;332 (5) :305 -311
52. Mundy GR. Cytokines and growth factors in the regulation of bone remodeling. J Bone Miner Res. 1993 ;8 (2) :S505 -S510
53. Arnaud CD. An integrated view of the role of the endocrine system in the genesis of the osteoporosis associanted with aging. Osteopros Int. 1993 ;1 (suppl) :S37 -S39
54. Simonet WS. et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997 ;89 :309 -19
55. Tsuda E. et al. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun. 1997 ;234 :137 -142
56. Sundeep K. The OPG/RANKL/RANK System. Endocrinology. 2001 ;142 (12) :5050 -5055
57. Jay A. et al. Infection by Human Immunodeficiency Virus - CD4 is Not Enough. N.Eng.J.Med. 1996 ;335 :1528 -1530
58. Feng Y. et al. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 ;272 :872 -876
59. Loetscher M. et al. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 1994 ;269 :232 -
60. Bleul C. et al. The lymphocyte chemo-attractant SDF-1 is a ligand for lestr/fusin and blocks HIV-1 entry. Nature. 1996 ;382 :829 -833
61. Oberlin E. et al. The CXC chemokine, stromal cell derived factor 1 (SDF-1), is the ligand for LESTR/fusin and prevents infection by lymphocyte-tropic HIV-1 syncytium-inducing strains. Nature. 1996 ;382 :833 -835
62. Cocchi F. et al. Identification of RANTES, MIP-1-alpha, and MIP-1-beta as the major HIV-suppressive factors produced by CD8(+) T cells. Science. 1995 ;270 :1811 -1815
63. Choe H. et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996 ;85 :1135 -1148
64. Kong YY. et al. Activated T cells regulated bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999 ;402 :304 -309
65. Alsina M. et al. Cytokine regulation of bone cell differentiation. Vitam Horm. 1996 ;52 :63 -98
66. Manolagas SD. Role of cytokines in bone resorption. Bone. 1995 ;17 (Suppl) :63S -67S
67. Roodman GD. Role of cytokines in the regulation of bone resorption. Calcif Tissue Int. 1993 ;53 (Suppl 1) :S94 -S98
68. Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996 ;11 :1043 -1051
69. Poli V. et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBOJ. 1994 ;13 :1189 -1196
70. Nguyen L. et al. Interleukin-1β stimulates bone resorption and inhibits bone formation in vivo. Lymphokine Cytokine Res. 1991 ;10 :15 -21
71. Panagakos FS. et al. Formation and mineralization of extracellular matrix secreted by an immortal human osteoblastic cell line: modulation by tumor necrosis factor-α. Inflammation. 1994 ;18 :267 -284
72. Dalgleish AG. The immune response to HIV: potential for immunotherapy? Immunol Today. 1995 ;16 :56 -58
73. Aukrust P. et al. Serum levels of tumor necrosis factor (TNF)α and soluble TNF receptors in human immunodeficiency virus type 1 infection-correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994 ;169 :420 -424
74. Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996 ;384 :529 -534
75. Matsuyama T. et al. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? AIDS. 1991 ;5 :1405 -1417
76. Hofbauer LC. et al. Endocrine implications of human immunodeficiency virus infection. Medicine. 1996 ;75 :262 -278
77. Besedovsky HO. et al. Immune-neuro-endocrine interactions: facts and hypothesis. Endocr Rev. 1996 ;17 :64 -102
78. DeLuca HF. Osteoporosis and the metabolites of vitamin D. Metabolism. 1990 ;39 (suppl 1) :3 -9
79. Heaney RP. et al. Menopausal changes in bone remodeling. J Lab Clin Med. 1978 ;92 :964 -970
80. Marcus R. Understanding osteoporosis. West J Med. 1991 ;15 :53 -60
81. Mazzuoli GF. et al. Pathogenetic aspects of involutional osteoporosis. Clin Rheumatol. 1989 ;8 (2) :22 -29
82. Eriksen EF. et al. Multiple sex steroid receptors in cultured human osteoblast-like cells. Jensen J, et al, eds. : International Symposium on Osteoporosis. 1987 :- Denmark. N.67
83. Bruera D. et al. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003 ;17 (13) :1917 -23
84. Glesby MJ. Bone disorders in human immunodeficiency virus infection. Clin Infect Dis. 2003 ;37 (Suppl 2) :S91 -5
85. Paton NIJ. et al. Bone mineral density in patients with human im-munodeficiency virus infection. Calcif Tissue Int. 1997 ;61 :30 -32
86. Lawal A. et al. Equivalent osteopenia in HIV-infected individuals studied before and during the era of highly active antiretroviral therapy. AIDS. 2001 ;15 :278 -80
87. McGowan I. et al. Assessment of bone mineral density (BMD) in HIV-infected antiretroviral-therapy-naive patients [abstract 628]. Program and abstracts of the 8th Conference on Retroviruses and Opportunistic Infection. 2001 Chicago
88. Knobel H. et al. Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS. 2001 ;15 :807 -8
89. Hernadez Quero J. et al. Alterations in bone turnover in HIV- positive patients. Infection. 1993 ;21 :220 -2
90. McNurlan MA. et al. Albumin synthesis and bone collagen formation in human immunodeficiency virus-positive subjects: differential effects of growth hormone administration. J Clin Endocrinol Metab. 1998 ;83 :3050 -5
91. Aukrust P. et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone- remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999 ;84 :145 -50
92. Serrano S. et al. Bone remodelling in human immunodeficiency virus-1-infected patients: a histomorphometric study. Bone. 1995 ;16 :185 -91
93. Mondy K. et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus infected individuals. Clin Infect Dis. 2003 ;36 :482 -90
94. Mora S. et al. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2001 ;15 :1823 -9
95. Schambelan M. et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA Panel. J Acquir Immune Defic Syndr. 2002 ;31 :257 -75
96. Baran DT. et al. Diagnosis and management of osteoporosis: guidelines for the utilization of bone densitometry. Calcified Tissue International. 1997 ;61 :433 -40
97. Miller PD. et al. Bone densitometry: the best way to detect osteoporosis and to monitor therapy. J Clin Endocrinol Metab. 1999 ;84 :1867 -71
98. Allison GT. et al. Osteonecrosis in HIV disease: epidemiology, etiologies, and clinical management. AIDS. 2003 ;17 (1) :1 -9
99. American College of Rheumatology Task Force on Osteoporosis Guidelines. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheum. 1996 ;39 (11) :1791 -801
100. European Foundation for Osteoporosis and the National Osteoporosis Foundation. Consensus development statement: who are candidates for prevention and treatment for osteoporosis? Osteoporos Int. 1997 ;7 :1 -6
101. Harris ST. et al. Alendronate use in postmenopausal women with low bone mass: combination with, comparison to, and use after discontinuation of hormone replacement therapy (HRT). J Bone Miner Res. 1999 ;14 (Suppl 1) :SU380 -
102. Black DM. et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996 ;348 (9041) :1535 -41
103. Weber TH. et al. Alendronate increases bone density in male idiopathic osteoporosis. J Bone Miner Res. 1999 ;14 (Suppl 1) :F345 - (Abstr)
104. Orwoll E. et al. Alendronate treatment of osteoporosis in men. J Bone Miner Res. 1999 ;14 (Suppl 1) :1205 - (Abstr)
105. Harris ST. et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999 ;282 (14) :1344 -52
106. National Osteoporosis Foundation. Physician's guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation. 1998
107. Reid D. et al. Risedronate is an effective and well-tolerated therapy in both the treatment and prevention of corticosteroid-induced osteoporosis. J Bone Miner Res. 1998 ;23 (5) :W464 - (Abstr)
108. Fosamax manufacturer's package insert information. Physicians' desk reference, 51st ed. Montvale, NJ: Medical Economics. 1997
109. Harris ST. et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999 ;282 (14) :1344 -52
110. Reid IR. Glucocorticoid osteoporosis--mechanisms and management. Eur J Endocrinol. 1997 ;137 (3) :209 -17
111. Healey JH. et al. A randomized controlled trial of salmon calcitonin to prevent bone loss in corticosteroid-treated temporal arteritis and polymyalgia rheumatica. Calcif Tissue Int. 1996 ;58 (2) :73 -80
112. Delmas PD. et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997 ;337 (23) :1641 -7
113. Ettinger B. et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999 ;282 (7) :637 -45
Author biography
K.R.S.Samabasiva Rao is Associate Professor and Head of Centre for Biotechnology at Acharya Nagarjuna University. He is specialized in the area Environmental Biotechnology and has been involved in teaching and research of Biochemistry. His major research interests are in the molecular diagnosis of pathological diseases, and role of anti-oxidants in diseased conditions.
N. Annapoorna did her MSc Biochemistry. She is presently working as Faculty at Centre for Biotechnology, Acharya Nagarjuna University and is pursuing her PhD in Clinical Biotechnology. Her research interest is on the side effects of Highly Active Antiretroviral Therapy (HAART).
G.Venkateswara Rao is a Faculty at Centre For Biotechnology, Acharya Nagarjuna University and is pursuing PhD in Biotechnology.
N.S. Reddy is a Faculty at Centre For Biotechnology, Acharya Nagarjuna University and is pursuing PhD in Biotechnology.
P. Rambabu is Professor at Government General Hospital and NTR University of Health Sciences, Vijayawada, A.P., India. He is a specialist clinician in the area of Diagnosis of Sexually Transmitted Diseases (STD). His major research interests are in the Molecular Diagnosis of AIDS, Diagnosis of side effects of anti retro-viral therapy.
![]() Corresponding address:
Corresponding address:
Dr. K.R.S.Samabasiva Rao. krssraocom. Phone - 91- 863- 2293400. Fax - 91-863-2293378

 Global reach, higher impact
Global reach, higher impact