3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(3):137-145. doi:10.7150/ijms.1.137 This issue Cite
Research paper
Anti-tumorigenic and Pro-apoptotic effects of CKBM on gastric cancer growth in nude mice
1 Department of Pharmacology, Faculty of Medicine, The University of Hong Kong, Hong Kong, HKSAR, China
2 CK Life Sciences Limited, Hong Kong, HKSAR, China
Received 2004-4-19; Accepted 2004-6-28; Published 2004-8-5
Abstract
Natural botanical products can be integrated with western medicine to optimize the treatment outcome, increase immune function and minimize the side effects from western drug treatment. CKBM is a combination of herbs and yeasts formulated based on traditional Chinese medicinal principles. Previous study has demonstrated that CKBM is capable of improving immune responsiveness through the induction of cytokine mediators, such as TNF-α and IL-6. In this study, we aimed to investigate the effect of this immunomodulatory drug on gastric cancer growth using a human xenograft model. Gastric cancer tissues were implanted subcutaneously into athymic nude mice followed by a 14-day or 28-day of CKBM treatment. Results showed that higher doses of CKBM (0.4 or 0.8 ml/mouse/day) produced a dose-dependent inhibitory effect on gastric tumor growth after 28-day drug treatment. This was associated with a decrease of cellular proliferation by 30% with concomitant increase in apoptosis by 97% in gastric tumor cells when compared with the control group. In contrast, CKBM showed no effect on angiogenesis in gastric tumors. This study demonstrates the anti-tumorigenic action of CKBM on gastric cancer probably via inhibition of cell proliferation and induction of apoptosis, and provides future potential targets of this drug candidate on cancer therapy.
Keywords: PCNA, apoptosis, angiogenesis, gastric cancer
1. Introduction
Natural botanical products have a long history in the world and are featured in using a complex combination of herbs to treat various diseases (e.g. rheumatoid arthritis, menopausal symptoms, colds and flu etc). CKBM-A01 is a combination of herbs including Wu Wei Zi (Schisandra chinensis), Ginseng (Panax ginseng), Hawthorn (Fructus Crataegi), Jujube (Ziziphus jujube), Soybean (Glycine Max) and Saccharomyces cerevisiae (baker's yeast), which is formulated based on traditional Chinese medicinal principles and processed by a proprietary technology developed by CK Life Sciences Limited (CKLS).
Schisandra chinensis has been shown to suppress the TPA-induced skin tumor formation and prostate carcinogenesis [1, 2]. It also aids to protect liver from injury, stimulates liver regeneration and reduces inflammation. Panax ginseng and Ziziphus jujube provide several pharmacological activities like modulating immune system and anti-stress activities [3]. The levels of cytokines (e.g. tumor necrosis factor-α, IL-1β, IL-6, interferon-γ) and reactive oxygen/nitrogen production were enhanced by these compounds to exert phagocytic activity [4]. Isoflavone-containing compound like soybean has been shown to lower estrogen levels, which helps to prevent and reduce the risk of breast cancer [5]. In addition, extracts of soybean suppressed F3II mouse mammary adenocarcinoma cell growth in vivo and in vitro through the G2/M cell cycle arrest [6].
In this study, we aimed to determine the effect of CKBM-A01 (a combined recipe of the above mentioned Chinese herbs and yeast) on the growth of human gastric cancer using a unique subcutaneous implantation model of gastric cancer tissue. In this connection, CKBM was given at different doses to investigate the effect on tumor growth. To further examine the anti-tumor mechanism, the three important biological parameters in tumorigenesis including cell proliferation and apoptosis [7], and also angiogenesis [8] were assessed in gastric tumors after drug treatment.
2. Materials and methods
Chemicals and reagents
CKBM-A01 (Batch no.: 0212201) was provided by CKLS (Hong Kong, China). The product is in liquid form. All chemicals and reagents were purchased from Sigma (Sigma Chemical Company, St Louis, USA) unless otherwise specified.
Cell culture
Human gastric carcinoma cell line (MKN-28) was purchased from RIKEN (The Institute of Physical and Chemical Research) Cell Bank, Japan. Cells were cultured in RPMI 1640 medium (GibcoBRL, USA) supplemented with 10% fetal bovine serum (BibcoBRL), 100 U/mL penicillin G, 100 µg/mL streptomycin, and maintained in a humidified 5% CO2 atmosphere at 37ºC.
Experimental animals and tumor implantation
Female athymic balb/c nude mice (Charles River Laboratories Ltd., USA), aged 4-6 weeks and weighing 19-24 g, were used in the tumor implantation model. They were housed in IVC cages of isolated ventilation to avoid microbial contamination. Gastric cancer tissues (1.5 mm3) were implanted subcutaneously (s.c.) into the right dorsal area of mice. Ten days after implantation, animals were randomized into 4 treatment groups: control group (0.8 ml distilled water/mouse) and CKBM-A01 (0.2, 0.4 or 0.8 ml/mouse) treatment groups. They were fed intragastrically (i.g.) daily for 14 and 28 days.
Tumor areas were measured every 7 days using a caliper, and the tumor area was calculated according to the formula: tumor volume (mm3) = d2 x D/2, where d and D were the shortest and the longest diameter respectively [9].
Assessment of cell proliferation
Paraffin-embedded tumor samples that had been fixed in formalin were cut into sections of 5 μm. Sections were incubated in citrate buffer (0.01 M, pH 6.0) at 80ºC for 15min and followed by digestion using pepsin (0.005%) in HCl (0.01 N, pH 2.0). After washing with PBS (0.01 M, pH 7.4), sections were incubated with normal serum (LSAB kit, DAKO, Copenhagan, Denmark) for 60 min. After blocking, they were incubated with a monoclonal primary antibody against mouse proliferating cell nuclear antigen (PCNA, 1:200) (Santa Cruz Biotechnology, Santa Cruz) for 90 min, and then incubated with Link reagent (LSAB kit, DAKO) for 60 min at room temperature. Sections were incubated with Streptavadin (LSAB kit, DAKO) for 60 min, and further incubated with hydrogen-peroxidase-diaminobenzidine (H2O2-DAB) to visualize the PCNA-positive cells. Finally, they were counterstained with Mayer's hematoxylin. The total number of proliferating cells in a total of ten fields (x 400) across and perpendicular at the center of the tumor was counted under the microscope. The results of cell proliferation were expressed as the number of PCNA-positive cells per field [10]. The same approach was adopted for measuring apoptosis and angiogenesis in the tumors.
Assessment of angiogenesis
Microvessel density was assessed by immunostaining as described previously [11] with modifications. Sections were digested with trypsin for 30 min at room temperature and incubated with a blocking agent (LSAB kit, DAKO, Copenhagen, Denmark) for 1 hr. They were then incubated with a rabbit anti-human von Willebrand factor (DAKO, Copenhagan, Denmark) with a dilution of 1:200 overnight at 4ºC. After washing with PBS (0.01 M, pH 7.4), sections were incubated with Link reagent (LSAB kit, DAKO) for 1 hr, followed by streptavidin (LSAB kit, DAKO) for another 1 hr. Sections were incubated with hydrogen-peroxidase-diaminobenzidine to visualize the microvessels. The results of microvessel density were expressed as the number of microvessel per field (x 200).
Assessment of apoptosis
Epithelial cell apoptosis was detected from paraffin-embedded tissue sections by the terminal deoxynucleotidyltranferase-mediated dUTP nick end–labeling (TUNEL) method as described previously [12]. Tissue sections were digested with 20 µg/ml proteinase K for 20 min at 37ºC and treated with 0.3% H2O2 solution at room temperature. Afterwards, they were incubated with TdT buffer containing 40 U/ml TdT and 5 nmol/ml biotinylated-dUTP, and kept in humidified atmosphere for 90 min at 37ºC. The reaction was quenched by washing with terminating buffer (300 mM sodium chloride and 30 mM sodium citrate) for 15 min. After blocking with normal serum, sections were incubated with peroxidase-labeled streptavidin for 30 min and stained with diaminobenzidine-H2O2 for 10 min. Finally, the sections were counterstained with Mayer's hematoxylin. For positive control, sections were treated with 0.5 U/ml DNase I in Dnase buffer (10 mM NaCl, 50 mM MnCl2, 0.1 mM CaCl2, 25 mM KCl and 10 mM Tris-HCl, pH 7.4) for 10 min; whereas negative control was prepared with the omission of TdT. The number of apoptotic cells in a total of ten fields (x 400) per tumor was counted under microscope. The results of apoptotic cells were expressed as the number of apoptotic cells per field.
Statistical analysis
Statistical significance between the treatment groups was analyzed using a two-way statistical analysis of variance (ANOVA), followed by Dunnett t-test and post-hoc analysis when necessary.
3. Results
Effect of CKBM treatment on gastric tumor growth
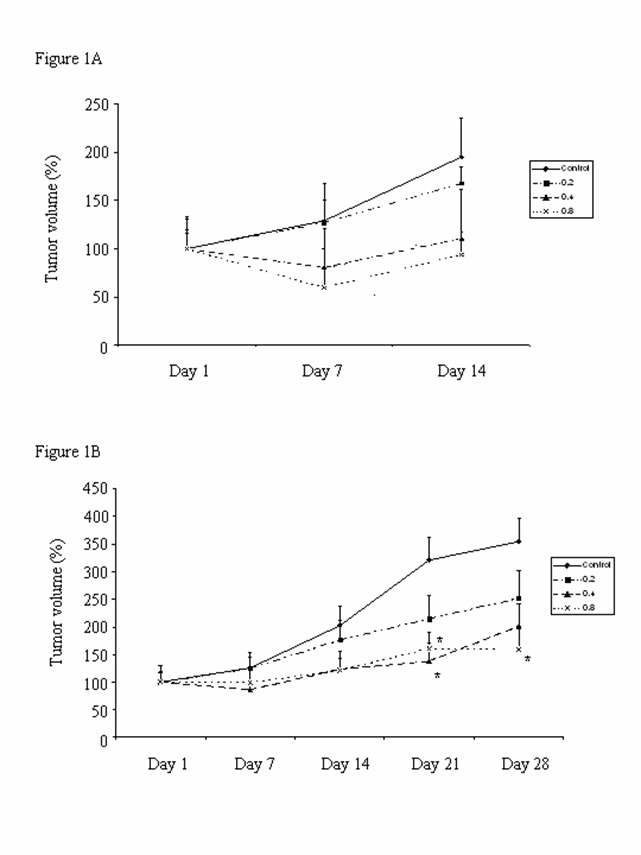
All the control (water vehicle-treated) and CKBM-treated mice developed subcutaneous tumor after gastric cancer tissue implantation. Figures 1A & 1B show the effect of CKBM on tumor growth 14 days or 28 days after treatment. The tumor growth of the control group increased steadily during the 14- and 28-day experimental periods. Results showed that CKBM exhibited a dose-dependent inhibition on tumor growth starting from Day 7 onwards. There was a significant 50% reduction on tumor growth in the CKBM-treated groups at doses of 0.4 and 0.8 ml/mouse on Day 21 and Day 28 after drug treatment (Figure 1B) when compared with the corresponding control group. CKBM did not affect the body weight changes in these animals during the experimental period.
Effect of CKBM treatment on angiogenesis in gastric tumors
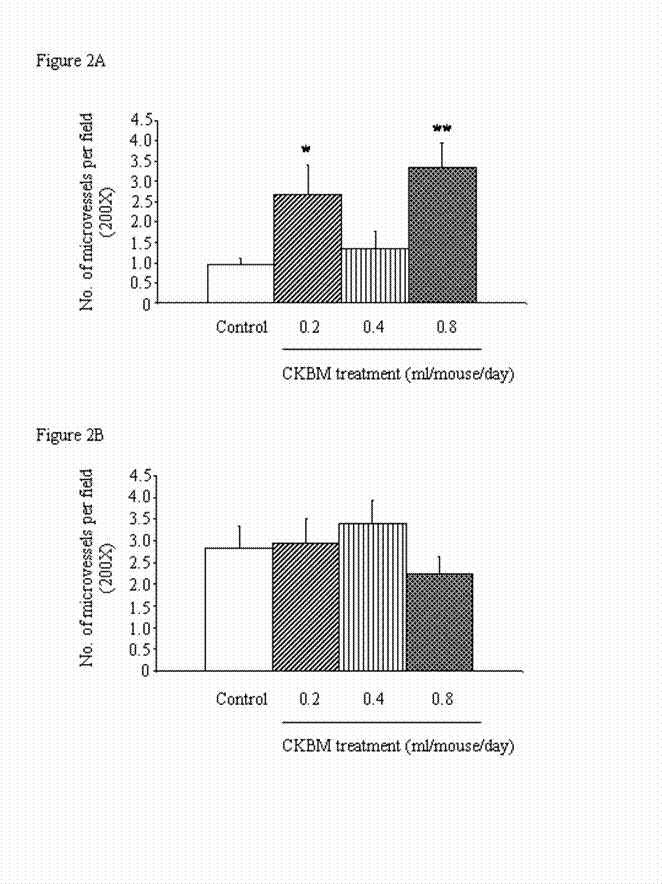
There was an increase in the microvessel density from Day 14 to Day 28 in the control group, implicating that angiogenesis is essential in the development of tumors (Figures 2A & 2B). However, CKBM treatment for 14 and 28 days did not produce dose-dependent effect on angiogenesis in gastric tumors. Notably, CKBM at all doses, except 0.4 ml/mouse, significantly increased angiogenesis in gastric tumors after 14 days of drug treatment (Figure 2A). In contrary, the stimulatory effect of CKBM on angiogenesis was not observed after 28 days of treatment although the highest dose of CKBM (0.8 ml/mouse) had a trend of decrease in angiogenesis but did not have statistical significance (Figure 2B).
Effect of CKBM treatment on apoptosis of cancer cells in gastric tumors
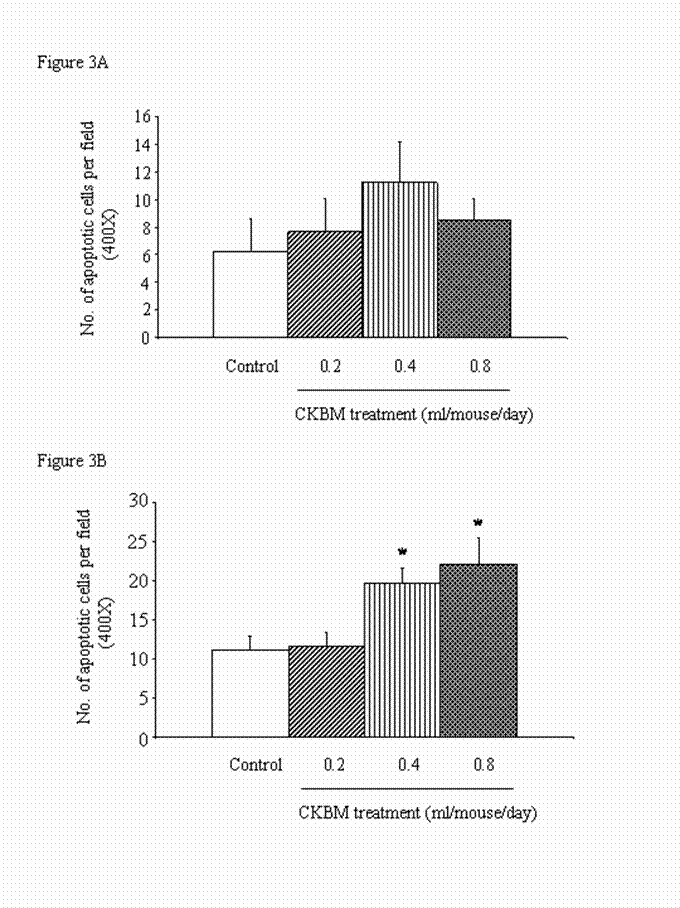
There was no significant effect on the number of apoptotic cells in the gastric tumors after 14 days of drug treatment (Figure 3A). However, prolonged treatment of CKBM to 28 days dose-dependently exhibited an induction of apoptosis in gastric cancer cells. At doses of 0.4 and 0.8 ml/mouse of CKBM treatment, apoptosis was significantly increased by 76% and 97% respectively when compared with the corresponding control group (Figure 3B).
Effect of CKBM treatment on cell proliferation of cancer cells in gastric tumors
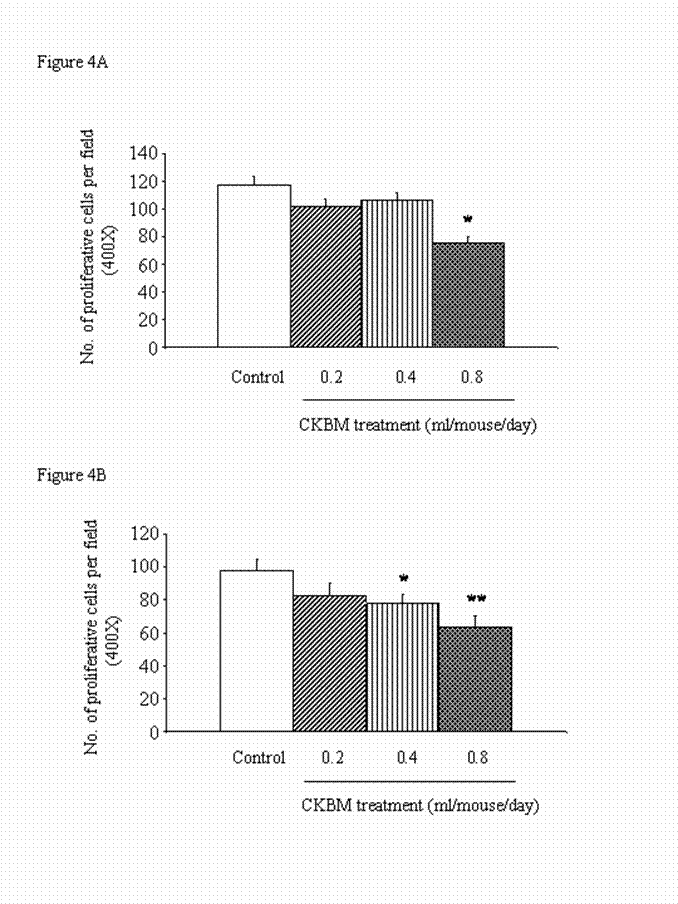
The anti-proliferative effect of CKBM treatment was observed as early as 14 days after drug treatment. The highest dose of 0.8 ml/mouse significantly reduced the number of cell proliferation in the gastric tumors by 30% when compared with the control group (Figure 4A). Similarly, prolong treatment of CKBM to 28 days dose-dependently inhibited the number of proliferative cells in gastric tumors (Figure 4B).
4. Discussion
In this study, CKBM significantly inhibited the growth of gastric tumor in human xenografts model using MKN-28 cells (Figures 1A & 1B). The efficacy of CKBM exhibited a dose-dependent manner in this ex-vivo model during the 28-day experimental period and exerted the inhibitory action as early as 21 days after drug treatment (Figure 1B). The effective doses of CKBM were found to be 0.4 and 0.8 ml/mouse, which significantly reduced the number of PCNA-positive cells and increased the apoptotic cells in the tumor tissues (Figures 3B & 4B). In contrast, CKBM did not affect angiogenesis at the time when it inhibited tumor growth (Figures 2A & 2B), although it increased with time along with tumor development in the control group. These findings implicated that CKBM suppressed gastric cancer growth specifically through the reduction of cell proliferation and promotion of apoptosis in this model.
Dietary supplement containing Schisandra chinensis has been shown to reduce prostate cancer cell growth and induce apoptosis by inhibiting androgen receptor expression [1]. Besides, soybeans contain various anti-carcinogenic compounds including lunasin and lectins that were shown to induce apoptosis in malignant cells [13]. As CKBM contains these compounds, this would partially explain why it inhibits tumor growth in the present animal model. Besides, CKBM may also inhibit cancer xenograft growth via cytokine secretion. Panax ginseng and Ziziphus jujube, the other major components in CKBM, induce cytokine release in macrophages [4]. Moreover, soluble β-glucans from Saccharomyces cerevisiae could activate macrophage to secrete TNF-α [14], which is found to have a pro-apoptotic effect on human cancer cells [15]. Anti-cancer drugs such as sulindac and its metabolite have been shown to augment TNF-α-mediated cell death signaling pathway through the blockade of NF-κB in human carcinoma cells [16]. Previous study also shows that CKBM is able to modulate the immune response through the induction of TNF-α and IL-6 secretion in peripheral blood mononuclear cells [17]. Although nude mice are immune deficient animals, there is evidence that cytokines are inducible in these animals. To this end, previous studies show that TNF-α can be induced and detected in the serum and splenocytes from nude mice after drug treatment such as 5-FU [18, 19, 20]. It is therefore possible that CKBM could induce cytokine release including TNF-α in these animals and inhibit tumor growth. However, further study is needed to determine how they are induced and the mechanisms they inhibit cancer cell growth in vivo.
In summary, CKBM is a mixture of natural herbs and yeast that is effective to reduce the growth of gastric tumors in nude mice. The anti-tumorigenic action of CKBM is perhaps through the induction of apoptosis and inhibition of proliferation of gastric cancer cells. However, further clinical trials in humans are needed to examine the pharmacokinetics and the therapeutic action of CKBM on cancer patients.
Competing interests
Edgar Shiu-Lam Liu, Ying-Jye Wu, and Shiu-Fun Pang work for CK Life Sciences Limited. Others: none declared.
References
1. Hsieh TC, Lu X, Guo J, Xiong W, Kunicki J, Darzynkiewicz Z, Wu JM. Effects of herbal preparation Equiguard on hormone-responsive and hormone-refractory prostate carcinoma cells: mechanistic studies. Int J Oncol. 2002 ;20 :681 -9
2. Yasukawa K, Ikeya Y, Mitsuhashi H, Iwasaki M, Aburada M, Nakagawa S, Takeuchi M, Takido M. Gomisin A inhibits tumor promotion by 12-O-tetradecanoylphorbol-13acetate in two-stage carcinogenesis in mouse skin. Oncology. 1992 ;49 :68 -71
3. Chang YS, Seo EK, Gyllenhaal C, Block KI. Panax ginseng: a role in cancer therapy? Integr Cancer Ther. 2003 ;2 :13 -33
4. Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002 ;24 :469 -82
5. Zhou JR, Yu L, Mai Z, Blackburn GL. Combined inhibition of estrogen-dependent human breast carcinoma by soy and tea bioactive components in mice. Int J Cancer. 2004 ;108 :8 -14
6. Hewitt AL, Singletary KW. Soy extract inhibits mammary adenocarcinoma growth in a syngeneic mouse model. Cancer Lett. 2003 ;192 :133 -43
7. Kim TI, Lee YC, Lee KH, Han JH, Chon CY, Moon YM, Kang JK, Park IS. Effects of nonsteroidal anti-inflammatory drugs on Helicobacter pylori-infected gastric mucosae of mice: apoptosis, cell proliferation, and inflammatory activity. Infect Immun. 2001 ;69 :5056 -63
8. Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003 ;37 :28 -33
9. Pratesi G, De Cesare M, Carenini N, Perego P, Righetti SC, Cucco C, Merlini L, Pisano C, Penco S, Carminati P, Vesci L, Zunino F. Pattern of antitumor activity of a novel camptothecin, ST1481, in a large panel of human tumor xenografts. Clin Cancer Res. 2002 ;8 :3904 -9
10. Liu ESL, Ye YN, Shin VY, Yuen ST, Leung SY, Wong BCY, Cho CH. Cigarette smoke exposure increases ulcerative colitis-associated colonic adenoma formation in mice. Carcinogenesis. 2003 ;24 :1407 -13
11. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N.Engl. J. Med. 1991 ;324 :1 -8
12. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 ;119 :493 -501
13. de Mejia EG, Bradford T, Hasler C. The anticarcinogenic potential of soybean lectin and lunasin. Nutr Rev. 2003 ;61 :239 -46
14. Lee DY, Ji IH, Chang HI, Kim CW. High-level TNF-α secretion and macrophage activity with soluble β-glucans from Saccharomyces cerevisiae. BioSci Biotechnol Biochem. 2002 ;66 :233 -8
15. Gillio Tos A, Cignetti A, Rovera G, Foa R. Retroviral vector-mediated transfer of the tumor necrosis factor alpha gene into human cancer cells restores an apoptotic cell death program and induces a bystander-killing effect. Blood. 1996 ;87 :2486 -95
16. Yasui H, Adachi M, Imai K. Combination of tumor necrosis factor-alpha with sulindac in human carcinoma cells in vivo. Ann N Y Acad Sci. 2003 ;1010 :273 -7
17. Chan BP, Yuen WF, Lee WH, Wong SN, Chung TY, Wu YJ, Pang SF. Immunomodulating effects of CKBM on the cytokine production in peripheral blood mononuclear cells (PBMCs) from healthy volunteers. Immunopharmacol Immunotoxicol. 2004 ;26 :177 -92
18. Lin LW, Lin XD, He YM, Gao SD, Xue ES. Experimental study on ultrasound-guided intratumoral injection of "Star-99" in treatment of hepatocellular carcinoma of nude mice. World J Gastroenterol. 2003 ;9 :701 -5
19. Nandakumar KS, Lakshmi Rao K, Pardhasaradhi BV, Khar A. Upregulation of antitumor immunity by IL-12 gene-transfected AK-5 tumor cells in vivo. Cytokines Cell Mol Ther. 1999 ;5 :7 -14
20. Okamoto M, Kasetani H, Kaji R, Goda H, Ohe G, Yoshida H, Sato M. cis-Diamminedichloroplatinum and 5-fluorouracil are potent inducers of the cytokines and natural killer cell activity in vivo and in vitro. Cancer Immunol Immunother. 1998 ;47 :233 -41
Figures
Effect of CKBM treatment (0.2, 0.4 or 0.8 ml/mouse, given i.g. once daily) for (A) 14 days; (B) 28 days on gastric tumor growth in Balb/c nude mice (*P<0.05 when compared with the corresponding control group). Values are means ± SEM of 10-14 mice per group.
Effect of CKBM treatment (0.2, 0.4 or 0.8 ml/mouse, given i.g. once daily) for (A) 14 days; (B) 28 days on number of blood vessels in gastric tumors (*P<0.05, **P<0.01 when compared with the corresponding control group). Values are means ± SEM of 4-14 mice per group.
Effect of CKBM treatment (0.2, 0.4 or 0.8 ml/mouse, given i.g. once daily) for (A) 14 days; (B) 28 days on number of apoptotic cells in gastric tumors (*P<0.01 when compared with the control group).. Values are means ± SEM of 4-13 mice per group.
Effect of CKBM treatment (0.2, 0.4 or 0.8 ml/mouse, given i.g. once daily) for (A) 14 days; (B) 28 days on number of proliferative cells in gastric tumors (*P<0.05, **P<0.01 when compared with the corresponding control group). Values are means ± SEM of 4-13 mice per group.
Author biography
Vivian Yvonne Shin (M. Phil.) is Ph.D. candidate in Department of Pharmacology, University of Hong Kong. Her current research includes identification of active components in cigarette smoke and signal transduction pathway in carcinogenesis of gastric cancer.
Wallace Hau-Leung So (B.Sc.) is technician in Department of Pharmacology, University of Hong Kong. He is actively involved in various areas of research including gastric ulceration and carcinogenicity.
Edgar Shiu-Lam Liu (Ph.D.) is Senior Science Officer of CK Life Sciences Limited, Hong Kong, China. He graduated from University of Toronto with a bachelor degree before completing Ph.D. study in Department of Pharmacology, University of Hong Kong. His research interests include drug development for immunity enhancement and cancer therapy.
Ying-Jye Wu (Ph.D.) is Technology Development Director of CK Life Sciences Limited. Dr. Wu has over 20 years of experience with US biomedical industry and is knowledgeable in the development of FDA-approved cancer, AIDS and hepatitis B products including proteomics-based diagnostic products for early cancer detection.
Shiu-Fun Pang (Ph.D.) is Vice President and Chief Technology Officer of CK Life Sciences Limited. Dr. Pang was the Head of Department of Physiology at University of Hong Kong prior joining the company. He had been the founding Editor and Editor-in-Chief of Biological Signals and Biological Signals and Receptors, Adjunct Professor of University of Toronto and is Honorary or Visiting Professor of over ten universities.
Chi-Hin Cho, PhD, is Chair Professor of Pharmacology at the University of Hong Kong. His research interests include drug development for peptic ulcer and inflammatory bowel diseases and pathogenesis of experimental ulcers, inflammation and cancer in the gastrointestinal tract. Prof. Cho has published more than 250 research peer-reviewed papers, 15 invited review articles and 14 book chapters. He is a Founding member or Executive Committee Member of the Hong Kong Pharmacology Society, International Conference on Ulcer Research, and the Gastrointestinal Pharmacology Section of International Union of Pharmacology. He serves on the editorial board of several journals such as European Journal of Pharmacology, Journal of Gastroenterology & Hepatology, and Journal of Pharmacological Sciences.
![]() Corresponding address:
Corresponding address:
Prof. C.H. Cho, Department of Pharmacology, The University of Hong Kong, Hong Kong, China. Tel: (852) 2819 9252, Fax: (852) 2817 0859, Email: chchohku.hk

 Global reach, higher impact
Global reach, higher impact