3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(3):126-136. doi:10.7150/ijms.1.126 This issue Cite
Research paper
HLA-DR regulation and the influence of GM-CSF on transcription, surface expression and shedding
1. Department of Immunology, University of Liverpool, Liverpool, UK.
2. Intensive Care Unit, Royal Liverpool University Hospital, Liverpool, UK.
3. Department of Clinical Chemistry, University of Liverpool, Liverpool, UK.
Received 2004-4-16; Accepted 2004-6-10; Published 2004-7-10
Abstract
Low surface HLA-DR expression is a feature in sepsis. However, the mechanisms that regulate HLA-DR expression have not been elucidated. The current study investigates regulation of HLA-DR gene transcription, post transcriptional events and shedding of surface HLA-DR, as well as the regulation of HLA-DR by GM-CSF and an immunomodulatory cytokine. Plasma and PBMC were collected from septic patients and healthy volunteers. An ELISA was developed to measure soluble HLA. PCR techniques were used to determine HLA-DR mRNA levels, and flow cytometry and fluorescent microscopy were used for measurement of surface expressed and intracellular HLA-DR. Septic patients fulfilling the criteria of the American College of Chest Physicians (ACCP) for sepsis were recruited for the study (n=70). HLA-DR was measured on three consecutive days, days seven and fourteen. Patients were excluded from the study if on immunosuppressive therapy. Results: Higher levels of shed HLA-DR were found in the plasma of septic patients compared to healthy controls. The level of HLA-DR mRNA was significantly lower in septic patients compared to healthy controls, however an increased intracellular HLA-DR expression was observed. When HL-60 cells were treated with GM-CSF, gene transcription, surface expression and shedding of HLA-DR were all up-regulated. These results indicate that the mechanisms involved in the regulation of HLA-DR in sepsis include shedding of HLA-DR from the cell surface and regulation of HLA-DR gene transcription. Post-translational processing of HLA-DR was also seen to be compromised. GM-CSF was shown to regulate HLA-DR at all these levels.
Keywords: Sepsis, HLA-DR, monocyte, GM-CSF, post translational
1. Introduction
Impaired monocyte function has been described in sepsis and has been characterised by inadequate respiratory burst, activation, antigen presentation and lowered expression of Human Leukocyte Antigen-DR (HLA-DR) [1;2] . HLA-DR is a glycosylated cell surface transmembrane protein expressed on antigen-presenting cells and constitutively expressed on monocytes. Expression of HLA-DR by monocytes is essential for the presentation of peptides derived from ingested microbes to CD4 positive T cells to initiate a specific immune response [3] . Low monocyte surface expression of HLA-DR is a feature of sepsis [3-8] , however the mechanisms for this reduced surface expression of HLA-DR have not been established. Possibilities include down regulation of gene transcription resulting in reduced mRNA production, and/or post-translational modification of HLA-DR affecting processing to the cell surface. Alternatively, HLA-DR could be cleaved from the monocyte surface and shed into the circulation in a soluble form. Higher levels of soluble HLA-DR (sHLA-DR) have been detected in plasma and synovial fluid in hyperinflammatory states [9] and in vitro experiments have shown that the inflammatory cytokine IFN-γ induced shedding of HLA-DR by human monocytes [10] . However whether this type of mechanism is responsible for depressed monocyte HLA-DR in sepsis is not established, as one report found plasma HLA-DR to be lower rather than raised in septic patients who had a down regulated surface expression [11] . Recently, the regulation of HLA-DR at the level of gene transcription has been investigated in patients with sepsis, this correlated with high cortisol levels and was thought to be acting on the down regulation of HLA-DR mRNA by also lowering the CIITA [12] IFN-γ has also been shown to regulate transcription of the HLA-DR gene on melanoma cell lines [13] . Recently a post-translational modification of HLA-DR was described in septic patients which may be responsible for the lowered monocyte surface expression of HLA-DR observed in these septic patients [14] .
Lowered HLA-DR expression has been associated with impaired monocyte function and restoring expression to normal levels has been proposed to be beneficial [3] . A candidate factor for activating monocytes and restoring functional indicators is granulocyte macrophage colony stimulating factor (GM-CSF). GM-CSF is a 22-kDa glycoprotein cytokine secreted by mononuclear leukocytes including monocytes. It functions as a growth and differentiation factor for immature phagocytic cells and clinically it is used to enhance reconstitution of the haemopoietic system, for example following chemotherapy [15] . In addition to this, GM-CSF has been demonstrated to enhance the capability of monocytes and macrophages to phagocytose invading pathogens [16] . GM-CSF treatment of septic patients' lymphocytes ex vivo has been shown to restore function in anergic monocytes [2] . It has been shown that GM-CSF induces monocyte activation [17] and can protect monocytes from becoming apoptotic [2] . Clinically, recombinant human GM-CSF has been shown to be effective in the treatment of neonatal sepsis [18] with neutropenia [19]
We have previously shown that monocyte HLA-DR surface expression was upregulated in short term culture with GM-CSF and that low plasma levels of GM-CSF were an indicator of poor prognosis in sepsis [20] . In the present study we have investigated whether GM-CSF could be a factor in regulating HLA-DR expression in PBMC from patients with sepsis.
2. Methods
Patient Selection
Ethical approval for the study was obtained from the Liverpool Research Ethics Committee. This was a single centre study at a medico-surgical adult Intensive Care Unit (ICU) of the Royal Liverpool University Hospital. All critically ill septic patients admitted to the ICU who were recruited were expected to stay in the ICU for at least 72 hours, not expected to die within 24 hours and fulfilled the consensus conference criteria for sepsis [21] . Assent was obtained from next of kin if patients themselves were unable to consent into the study. Patients were excluded from the study if any of the following criteria were applicable: patients who were septic for greater than 72h; patients who were immunosuppressed due to prednisolone therapy >5 mg per day or equivalent, (excluding inhaled steroids); patients with clinically suspected or confirmed acquired immunodeficiency syndrome, hepatitis B or C; granulocyte count less than 1000 per mm3 due to a cause other than severe sepsis. Also patients with an underlying medical condition, exclusive of septic shock which was expected to cause death within three months from study entry were excluded. Healthy volunteers (n=45) were included as controls. The control group consisted of laboratory staff, median age 32. Mortality and focus of sepsis are described in the previous study [22] .
Sample preparation and flow cytometry
Arterial blood was collected from septic patients on the day sepsis was diagnosed (day 0) and on days 3, 7, and 14 for PCR studies and days 0, 1, and 2 for measurement of soluble HLA-DR. Blood (7.5 ml) was collected in preservative free heparin (final concentration of 100 iu/ml) and cells were prepared immediately. PBMC were isolated by density centrifugation using Ficoll Paque (Pharmacia Biotech, Sweden). Briefly, whole blood was layered onto an equal volume of Ficoll and centrifuged at 400xg for 20 minutes at 18˚C. Following isolation and washing of the PBMC viability was assessed to greater than 95% using trypan blue exclusion and the PBMC were adjusted to a concentration of 1x106 PBMC/ml.
Flow cytometric analysis was carried out on Coulter Epics Flow Cytometer. PBMC were dual-labelled with a mouse IgG anti-human HLA-DR with a fluorescein isothiocyanate (FITC) conjugate, clone L243 (Becton Dickinson, UK) and with a mouse IgG anti-human CD14 R-phycoerythrin conjugate (R-PE), clone M5E2, (Becton Dickinson, UK) to identify monocytes. Briefly 100 μl of cells were incubated with 5 μl of neat antibody for 30 minutes at 4˚C in the dark, washed and resuspended in 0.5 ml of PBS. For intracellular measurement of HLA-DR the PBMC were fixed for 10 minutes in 1% paraformaldhyde, washed in PBS and then incubated in saponin buffer (0.04% EGTA; 0.02% NaN3; 1% heat inactivated FCS; 0.1% saponin in PBS) before incubation with antibody as above. [All products from Sigma, UK unless mentioned otherwise]. Both the percentage of cells expressing HLA-DR on monocytes and the density of HLA-DR was measured.
Immuno-fluorescent microscopy of HLA-DR in PBMC
Cytospins of PBMC obtained from healthy controls and septic patients were used for observation of intracellular and surface staining of HLA-DR. The cytospins were fixed with 4% paraformaldehyde (Sigma, UK) and, after a brief wash in PBS, for those samples which required permeabilisation, the slides were immersed in methanol (which had been cooled for 1 hour at –20ºC, and incubated at –20ºC for 5-10 minutes. Following washing, the slides were incubated in the dark for 40 minutes at room temperature with the mouse IgG anti-human HLA-DR-FITC. Slides were washed thoroughly and a fluorescent mounting medium was added (Vectorshield, Vector Labs, UK) .Coverslips were placed and sealed with nail hardener and viewed by fluorescent microscopy (BioRad, UK).
RNA preparation and polymerase chain reaction (PCR)
From the isolated PBMC, 1x106 cells were resuspended in TRIzol (Gibco Life Technologies, UK) and RNA extracted following manufacturer's directions (Invitrogen, UK). Reverse transcription was carried out using ImpromII reverse transcriptase (Promega, UK) on 1 μl of RNA following manufacturer's directions to make a cDNA template. The reverse transcribed product (1μl) was used for PCR. Primers were designed complementary to a 404 base pair product in the non-polymorphic region of the alpha chain of HLA-DR. For each sample, two reactions were set up; one containing reverse transcriptase positive product and one reverse transcriptase negative product. This allowed identification of DNA contamination. The sequence of the amplified primer product revealed homology to the primer target sequence. The primers were designed using Primer Select software (DNA Star).
Forward HLA-DR Primer: ATCATGACAAAGCGCTCCAACTAT
Reverse HLA-DR Primer: GATGCCCACCAGACCCACAG (Sigma, UK)
Soluble HLA-DR measurement by ELISA
Ninety six well ELISA plates (Nunc, Denmark) were coated overnight at 4˚C with a monoclonal mouse anti-human HLA-DR antibody, clone G46-6 (Pharmingen, UK) at a concentration of 1 μg/ml in 0.05M carbonate buffer, pH 9.6. Following washing in PBS Tween, protein binding sites were blocked with 1% skimmed milk powder in PBS, and the washing step repeated.
Standards (purified HLA-DR) were kindly donated by Dr S Jurcevic. Standard concentrations began at 2.5μg/ml, as this was the upper limit concentration in patient samples or in the supernatant of cultured HL-60 cells with GM-CSF. Samples were added undiluted and at 1 in 10 dilution, in duplicate. Following an incubation and washing, the primary detection antibody was added, a mouse anti human IgG1 anti-HLA-DR (CR3/43) (Dako, Denmark), at a concentration of 1 μg/ml. After a 2h incubation at room temperature, plates were washed and the secondary detection antibody was added, 100μl of biotinylated anti-mouse IgG1 antibody at a concentration of 2ug/ml (Pharmingen, UK). Following a 2h incubation plates were washed and 100μl of avidin peroxidase conjugate (1/1000) was added to each well and incubated for 30 minutes at room temperature. Plates were washed thoroughly, then 50μl per well of chromogen/substrate (0.3mg/ml of 2, 2'-azino- bis [3-ethylbenzthiazoline-6-sulfonic acid] containing 0.02% hydrogen peroxide in 0.1M citrate buffer pH 6.0, [ABTS] Sigma, UK) was added following manufacturer's directions and incubated at room temperature until colour development. Absorbance was then read at 405 nm on an ELISA plate reader.
HL-60 cell line maintenance and culture
HL-60 cells, a human promyelocytic cell line, were maintained in complete RPMI (10% FCS, 20mM L-glutamine, 20U/ml penicillin, 100ug/ml streptomycin). HL-60 cells (5 x 105 cells/ml) were cultured with GM-CSF at concentrations of 1ng/ml and 10ng/ml At time points 4, 6, and 8 hours, cells were evaluated for surface expression of HLA-DR and HLA-DR mRNA production. Control samples were included where cells were incubated in complete RPMI 1640 medium alone.
HL-60 cells (200 μl) were taken from the well, pelleted down at 5000 x g for 30 seconds, washed twice in PBS, then incubated with 2.5 μl of PE labelled anti-human HLA-DR antibody (Pharmingen, UK) as already described above. HLA-DR mRNA was measured as described previously. Supernatants from the cell cultures with GM-CSF were stored at –70ºC and assayed for sHLA-DR as described earlier.
Western Blotting for sHLA-DR in cell lysates and plasma
Cell lysates of protein were prepared from PBMC of septic patients and healthy volunteers. Protein was extracted by resuspended 2x106 PBMC in protein lysis buffer (50mM HEPES [pH 7.2], 150mM NaCl, 5mM EDTA, 0.5% NP-40) containing a cocktail of mammalian protease inhibitors (Sigma, UK). The PBMC were centrifuged after a 10 minute incubation on ice and the resultant supernatant contained the protein extract.
HLA-DR standard, cell lysates and plasma samples were separated using a Mini-PROTEAN® II. The separation gel was a 14% polyacrylamide gel (National Diagnostics, Georgia) prepared following manufacturer's directions.
The cells lystes were used at a 1 in 10 dilution, plasma samples were run neat in the native, unreduced, state. Standard molecular weight markers were run, (132, 90, 55, 43, 34, 23) (Cruz marker, Santa Cruz Biotech, CA). After SDS-PAGE, transfer of proteins was carried out using the Mini Trans-Blot® Electrophoretic Transfer Cell (Bio-Rad, UK) and the membrane was blocked (5% skimmed milk powder, 0.05% Tween in TBS). A monoclonal mouse IgG1anti human anti-HLA-DR [CR3/43] was added at a concentration of 1ug/ml (Dako, Denmark), the membranes were then washed, and incubated with the secondary detection antibody, a Cruz Marker-Compatible Secondary Antibody (Santa Cruz Biotech, CA), an HRP conjugated goat anti mouse IgG for HLA-DR detection. The membranes were developed using Western Blotting Luminol Reagent (Santa Cruz Biotech, CA) followed by exposure to Hyperfilm (Amersham, UK).
Statistical analysis
The Shapiro Wilk test was applied to test for normally distributed data. For non-parametric data, the Mann-Whitney U test was applied to compare two independent samples when test data were compared directly with control data. The Kruskall Wallis one-way ANOVA was applied to data where more than two independent samples were compared with each other. The Friedman test and the R2 correlation coefficient was applied to identify significant trends. In normally distributed paired sets of data the paired Students T Test was applied.
3. Results
PCR analysis of HLA-DR mRNA in septic patients versus healthy controls
HLA-DR mRNA was measured in 8 healthy controls by PCR. The mRNA bands were semi-quantitated using densitometry software which gave a numerical value.
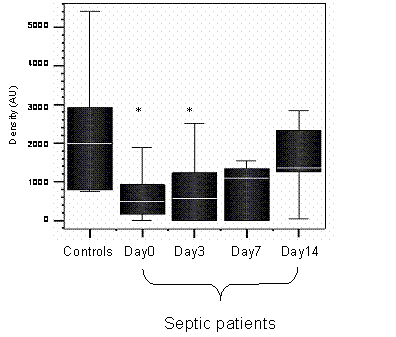
HLA-DR mRNA was measured in the septic patients, on days 0, 3, 7 and 14, if patients survived until day 14. The densitometry values for mRNA of the healthy controls were compared to the values of the septic patients on each day. Figure 1 shows HLA-DR mRNA for septic patients monocytes was significantly lower than in healthy controls (day 0 p = 0.02, day 3 p= 0.05).
Intracellular expression of HLA-DR in PBMC of healthy controls and septic patients
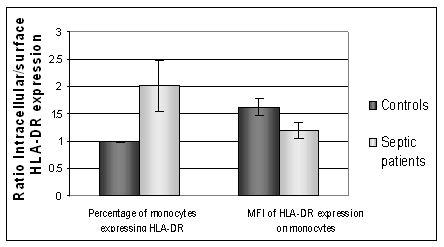
Intracellular HLA-DR was measured in permeabilised cells to investigate the presence of HLA-DR retained within the PBMC population. Flow cytometric measurement of HLA-DR expression in permeabilised cells measures both intracellular and surface. A comparison to the surface value allowed an estimate of intracellular versus extracellular expression of HLA-DR. Healthy controls (n=6) had lower expression of intracellular HLA-DR than septic patients. Figure 2 shows the intracellular and surface expression of HLA-DR from septic patients and healthy controls. In septic patients the intracellular expression was significantly higher than the corresponding surface expression, P=0.01. For healthy controls the level of intracellular expression was not statistically different to the surface expression, p=0.85, (paired Students T Test). Septic patients had a higher intracellular expression of HLA-DR than healthy controls.
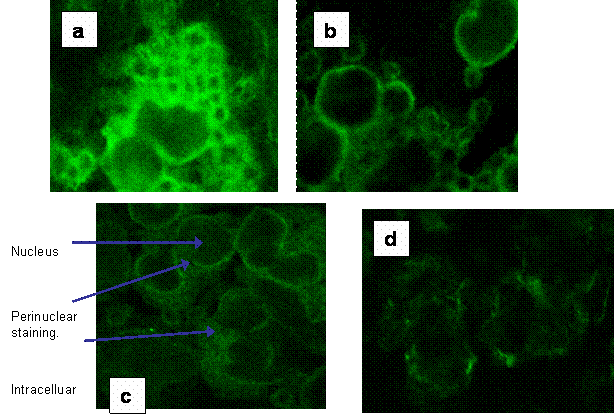
Fluorescent microscopy was used with confocal imaging to allow visualisation of sections through the cell to observe surface and intracellular staining. Figures 3a-d show the intracellular and surface staining of PBMC in a healthy control and from a septic patient. These images illustrated a high intracellular expression of HLA-DR within the PBMC in septic patients where surface expression was barely visible.
Plasma sHLA-DR levels in septic patients versus healthy controls
When sHLA-DR was measured in the plasma, healthy controls had a lower levels than both septic survivors and non-survivors, p =0.014, (Kruskall Wallis Test for 3 independent samples (Figure 4). The septic survivors showed a trend of higher levels of sHLA-DR than the non-survivors when the sHLA-DR was measured over the three days, but this was not statistically significant.
HL-60 culture with GM-CSF
HL-60 cells were used as an in vitro model to study the effects of GM-CSF on HLA-DR expression. HL-60 cells constitutively expressed surface HLA-DR which was measured using flow cytometry as previously described. GM-CSF did not significantly affect HLA-DR expression at 4 or 8 hours incubation.
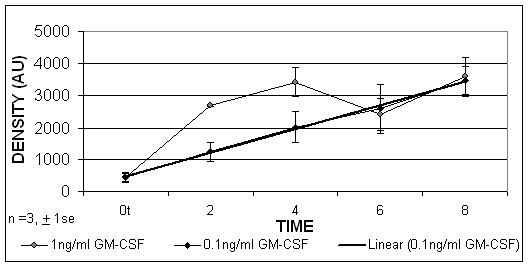
Using limited dilution PCR techniques it was found that, when the HL-60 cells were cultured in the presence of GM-CSF, the HLA-DR mRNA was increased after 2, 4, 6 and 8 hours. These results indicated that GM-CSF was able to upregulate significantly HLA-DR expression at the level of gene transcription, which was detectable from 2 hours, p=0.02. (Figure 5).
When the supernatants of the HL-60 cells, which were cultured with GM-CSF, were assayed for sHLA-DR there was a significant dose dependent increase in sHLA-DR, p=0.04 Friedman test. (Figure 6).
4. Discussion
A lowered expression of both the percentage of monocytes expressing HLA-DR and the density of HLA-DR expression have been observed as a feature of sepsis [4;5;7;8;22-25] . Only recently have the possible mechanisms for lowered HLA-DR expression been investigated. Ditschkowski et al. (1999) investigated the presence of circulating sHLA-DR in sepsis and suggested that shedding of HLA-DR could be responsible for the lowered expression of HLA-DR. Fumeaux et al. (2002) have recently described a post-translational modification of HLA-DR which may be responsible for a lowered cell surface expression of HLA-DR preventing expression to the cell surface and leading to accumulation in the cytoplasm. In this study it was also proposed that the cytokine which may be mediating this could be IL-10, and that the transcriptional expression of HLA-DR mRNA was not altered in sepsis. In the present study, it has been demonstrated that the transcription of HLA-DR in septic patients was significantly lowered early in sepsis (days 0 and 3) compared with healthy control levels. Interestingly the levels started to return closer to normal levels by day 14. The effect of GM-CSF on this transcriptional regulation was investigated. We had previously demonstrated GM-CSF could upregulate surface expression of HLA-DR in septic patients' monocytes and we also showed that poor prognosis in these patients was associated with low plasma GM-CSF [20] . As GM-CSF has been suggested to be an important immunomodulatory cytokine in sepsis, we investigated its action on regulation of HLA-DR at the level of transcription and shedding. Recombinant human GM-CSF has been shown to reduce mortality in neonatal sepsis with neutropenia [19] and in vitro work has demonstrated that GM-CSF was able to restore or boost the function of human monocytes [18]. For example GM-CSF has been shown to stimulate monocyte TNF and TNF-receptor secretion [27] . GM-CSF has also been shown to normalise monocyte HLA-DR expression in septic patients with low monocyte HLA-DR expression [2] and septic neonates [18], as well as enhancing production of monocyte reactive oxygen species [17] . When the cell line, HL-60, was cultured with GM-CSF we demonstrated that an up-regulation of HLA-DR mRNA was observed from 2 hours culture and at time points 4, 6 and 8 hours. Transcriptional regulation of HLA-DR by IFN-γ has previously been reported [10] and the present study indicates that GM-CSF is also able to regulate HLA-DR at the level of transcription.
To investigate whether a post-translational modification was a mechanism involved in the regulation of HLA-DR, as described in the study of Fumeaux et al. (2002), flow cytometry and immunoflourescence were used to determine intracellular HLA-DR expression in healthy and septic patients. To investigate whether high levels of HLA-DR were expressed intracellulary in septic patients, patients were selected having a low surface expression (<30% of monocytes expressing HLA-DR). It was demonstrated in septic patients, that a higher number of cells expressed intracellular rather than surface cell surface HLA-DR. On the cytospins, it was further demonstrated that there was high intracellular HLA-DR expression compared with the respective surface expression. Our results support the observations seen in the study of Fumeaux et al. (2002) demonstrating that a modification of HLA-DR, post-translationally, may be one mechanism responsible for the low surface expression of monocyte HLA-DR.
When shed sHLA-DR was measured in plasma, significantly higher levels were observed in the septic patients, compared with healthy controls. This suggests that HLA-DR may be shed in sepsis. These results were in contrast to a previous study [11] which reported a significantly lower concentration of plasma sHLA-DR in septic patients when compared with non-septic ICU patients. However, in this study, no healthy controls were included, a normal level was not established and it was possible that both groups had high sHLA-DR levels. As a normal level was not established it is not possible to directly compare those results with our own. However, in the non-septic ICU patients in that study, [11] the concentrations of sHLA-DR were over 1ug/ml, whereas in our patients and controls we never found concentrations over 0.5ug/ml. In our study, higher levels have been observed in the plasma of septic patients, however this cannot be directly linked to this being shed from the surface of monocytes, as B cells, activated T cells and dendritic cells also express HLA-DR on their surface [3].
To assess the relative contribution of shed to total HLA-DR, the sHLA-DR in the plasma was compared to the total HLA-DR present in cell. The level in the plasma (40ul neat) was far less than the level of HLA-DR in the cell lysates (5ul of a 1 in 10 dilution, from 2 x 106 PBMC). The shed HLA-DR therefore seemed to represent a small fraction of the total HLA-DR of the cell, however the function of these shed HLA-DR molecules remains to be investigated with respect to any potential role in sepsis.
Serum sHLA-DR has been reported to be increased in infections and in inflammatory diseases or diseases with an activated immune profile. For example, in rheumatoid arthritis, sHLA-DR in synovial fluid correlated with disease activity [27; 28] . Raised synovial fluid sHLA-DR has also been reported to be associated in joint inflammatory disease [9] and high levels of sHLA-DR have been associated with viral infection [29] . The high levels of sHLA-DR in septic patients compared with healthy controls may therefore be indicative of infection and their response to inflammation. Further evidence for this was the dose- dependant increase in shedding of HL-60 HLA-DR upon incubation with the inflammatory mediator GM-CSF.
The present study has elucidated some of the mechanisms by which HLA-DR expression in regulated in sepsis. The demonstration that GM-CSF increased the shedding of HLA-DR from the cell surface and also increased HLA-DR gene transcription may have implications for the therapy of septic patients with GM-CSF as these trials have already begun [18;19;30;31] . Our findings are also relevant to the understanding of the mechanisms through which clinical improvement through such immunomodulatory therapies may occur.
Acknowledgements
We would like to thank Bio Products Laboratory (BPL) and the Royal Liverpool & Broadgreen University Hospital NHS Trust (RLUHT) and acknowledge their financial support of this study, project number 1576. We would also like to thank Dr Clive Dash Medical Director of BPL for his his help and support, the staff of the ITU at the RLUHT in particular the research nurses, Neal Gratton and Andrea Fazakerley for recruiting patients into the study. We would also like to acknowledge Dr S Jurcevic for the kind gift of the purified HLA-DR, Drs B Flanagan and D Newton for their advice and technical assistance with the PCR assays and Western Blotting.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Haveman JW, Muller Kobold AC, Tervaert JW, van den Berg AP, Tulleken JE, Kallenberg CG. The central role of monocytes in the pathogenesis of sepsis: consequences for immunomonitoring and treatment. Neth.J.Med. 1999 ;55 :132 -41
2. Williams MA, Withington S, Newland AC, Kelsey SM. Monocyte anergy in septic shock is associated with a predilection to apoptosis and is reversed by granulocyte-macrophage colony-stimulating factor ex vivo. J.Infect.Dis. 1998 ;178 :1421 -33
3. Cheadle WG. The human leukocyte antigens and their relationship to infection. Am.J.Surg. 1993 ;165 :75S -81S
4. Volk HD, Thieme M, Heym S, Döcke WD, Ruppe U, Tausch W, Manger D, Zuckermann S, Golosubow A, Nieter B. Alterations in function and phenotype of monocytes from patients with septic disease--predictive value and new therapeutic strategies. Behring Inst.Mitt. 1991 ;88 :208 -15
5. Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat.Med. 1997 ;3 :678 -81
6. Cheadle WG, Hershman MJ, Wellhausen SR, Polk HC Jr. HLA-DR antigen expression on peripheral blood monocytes correlates with surgical infection. Am.J.Surg. 1991 ;161 :639 -45
7. Asadullah K, Woiciechowsky C, Docke WD, Egerer K, Kox WJ, Vogel S, Sterry W, Volk HD. Very low monocytic HLA-DR expression indicates high risk of infection-- immunomonitoring for patients after neurosurgery and patients during high dose steroid therapy. Eur.J.Emerg.Med. 1995 ;2 :184 -90
8. Döcke WD, Syrbe U, Meinicke A, Platzer C, Makki A, Asadullah K, Klug C, Zuckermann H, Reinke P, Brunner H, Von Baehr R, Volk HD. Improvement of monocytic function - a new therapeutic approach in sepsis? In: (ed.) Reinhart et al. Update in intensive care medicine. New York: Springer. 1994 :473-488
9. Claus R, Bittorf T, Walzel H, Brock J, Uhde R, Meiske D, Schulz U, Hobusch D, Schumacher K, Witt M, Bartel F, Hausmann S. High concentration of soluble HLA-DR in the synovial fluid: generation and significance in "rheumatoid-like" inflammatory joint diseases. Cell Immunol. 2000 ;206 :85 -100
10. Gershon HE, Kuang YD, Scala G, Oppenheim JJ. Effects of recombinant interferon-gamma on HLA-DR antigen shedding by human peripheral blood adherent mononuclear cells. J.Leukoc.Biol. 1985 ;38 :279 -91
11. Ditschkowski M, Kreuzfelder E, Rebmann V, Ferencik S, Majetschak M, Schmid EN, Obertacke U, Hirche H, Schade UF, Grosse-Wilde H. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann.Surg. 1999 ;229 :246 -54
12. Le Tulzo Y, Pangault C, Amiot L, Guilloux V, Tribut O, Arvieux C, Camus C, Fauchet R, Thomas R, Drenou B. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. 2004 ;169 (10) :1144 -51
13. Rosa F, Hatat D, Abadie A, Wallach D, Revel M, Fellous M. Differential regulation of HLA-DR mRNAs and cell surface antigens by interferon. EMBO J. 1983 ;2 :1585 -9
14. Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am.J.Respir.Crit Care Med. 2002 ;166 :1475 -82
15. Williams MA, Kelsey SM, Collins PW, Gutteridge CN, Newland AC. Administration of rHuGM-CSF activates monocyte reactive oxygen species secretion and adhesion molecule expression in vivo in patients following high-dose chemotherapy. Br.J.Haematol. 1995 ;90 :31 -40
16. Mellstedt H, Fagerberg J, Frodin JE, Henriksson LL, Hjelm-Skoog A, Liljefors M, Ragnhammar P, Shetye J, Osterborg A. Augmentation of the immune response with granulocyte-macrophage colony- stimulating factor and other hematopoietic growth factors. Curr.Opin.Hematol. 1999 ;6 :169 -75
17. Flohe S, Borgermann J, Dominguez FE, Majetschak M, Lim L, Kreuzfelder E, Obertacke U, Nast-Kolb D, Schade FU. Influence of granulocyte-macrophage colony-stimulating factor (GM-CSF) on whole blood endotoxin responsiveness following trauma, cardiopulmonary bypass, and severe sepsis. Shock. 1999 ;12 :17 -24
18. Drossou-Agakidou V, Kanakoudi-Tsakalidou F, Sarafidis K, Tzimouli V, Taparkou A, Kremenopoulos G, Germenis A. In vivo effect of rhGM-CSF And rhG-CSF on monocyte HLA-DR expression of septic neonates. Cytokine. 2002 ;18 (5) :260 -5
19. Bilgin K, Yaramis A, Haspolat K, Tas MA, Gunbey S, Derman O. A randomized trial of granulocyte-macrophage colony-stimulating factor in neonates with sepsis and neutropenia. Pediatrics. 2001 ;107 :36 -41
20. Perry SE, Mostafa SM, Wenstone R, McLaughlin PJ. Low plasma granulocyte-macrophage colony stimulating factor is an indicator of poor prognosis in sepsis. Intensive Care Med. 2002 ;28 :981 -4
21. Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992 ;101 :1481 -3
22. Perry SE, Mostafa SM, Wenstone R, Shenkin A, McLaughlin PJ. Is low monocyte HLA-DR expression helpful to predict outcome in severe sepsis? Intensive Care Med. 2003 ;29 :1245 -52
23. Asadullah K, Woiciechowsky C, Docke WD, Liebenthal C, Wauer H, Kox W, Volk HD, Vogel S, Von Baehr R. Immunodepression following neurosurgical procedures. Crit Care Med. 1995 ;23 :1976 -83
24. Volk HD, Reinke P, Docke WD. Immunological monitoring of the inflammatory process: Which variables? When to assess? Eur.J.Surg.Suppl. 1999 :70-2
25. Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller J.M, Döcke WD, Kox WJ. Monocyte deactivation--rationale for a new therapeutic strategy in sepsis. Intensive Care Med. 1996 ;22 (Suppl 4) :S474 -S481
26. Williams MA, Kouroumoussis I, Syndercombe-Court D, Hendry L, Newland AC, Kelsey SM. Administration of recombinant human granulocyte-macrophage colony- stimulating factor after chemotherapy regulates the expression and secretion of monocyte tumor necrosis factor (TNF) and TNF receptors p55 and p75. Blood. 1993 ;86 :4234 -42
27. Verbruggen L, Versaen H, Rebmann V, Duquet W, De Cock S, Grosse-Wilde H, Demanet C. Soluble HLA-DR levels in serum are associated with therapy and genetic factors in rheumatoid arthritis. Hum.Immunol. 2002 ;63 :758 -
28. Verbruggen LA, Dumarey N, Van de Velde H, V Rebmann V, Flament J, Van Wayenberge C, Grosse-Wilde HC, Demanet C. Soluble HLA-DR antigen levels in serum correlate with rheumatoid arthritis disease activity and the presence of disease-associated epitopes. Tissue Antigens. 2000 ;56 :436 -40
29. Filaci G, Contini P, Brenci S, Lanza L, Scudeletti M, Indiveri F, Puppo F. Increased serum concentration of soluble HLA-DR antigens in HIV infection and following transplantation. Tissue Antigens. 1995 ;46 :117 -23
30. Cairo MS, Christensen R, Sender LS, Ellis R, Rosenthal J, van de Ven C, Worcester C, Agosti JM. Results of a phase I/II trial of recombinant human granulocyte- macrophage colony-stimulating factor in very low birthweight neonates: significant induction of circulatory neutrophils, monocytes, platelets, and bone marrow neutrophils. Blood. 1995 ;86 :2509 -15
31. Miura E, Procianoy RS, Bittar C, Miura CS, Mello C, Christensen RD. A randomized, double-masked, placebo-controlled trial of recombinant granulocyte colony-stimulating factor administration to preterm infants with the clinical diagnosis of early-onset sepsis. Pediatrics. 2001 ;107 :30 -5
Figures
Densitometry plot of the HLA-DR mRNA of healthy controls and septic patients. * denotes significance between septic patients and healthy controls on days 0 and 3, p < 0.05, Mann Whittney U test. n=12 (septic patients) n=8 (healthy controls)
The intracellular: surface expression ratio of HLA-DR. A value above 1 shows a higher intracellular expression of HLA-DR compared to surface expression. Septic patients show a significantly higher number of PBMC expressing HLA-DR when intracellular expression is measured, compared to surface expression, p=0.01. (MFI: Median Fluorescent intensity)
(a) Fluorescent image of intracellular staining of a section through PBMC from a healthy control for HLA-DR expression. (b) The fluorescent image of a section though healthy control PBMC showing the surface expression of HLA-DR. (c) Fluorescent image of intracellular staining for HLA-DR of a section through PBMC from a septic patient. (d) shows the weak surface staining of HLA-DR in the same patients' PBMC.
Soluble HLA-DR in plasma from healthy controls and septic patients, divided into survivors and non-survivors, measured on the day 0 of the diagnosis of sepsis. P=0.014, healthy controls versus survivors and non-survivors (Kruskall Wallis test for 3 independent samples).

The PCR results expressed by densitometry values of HL-60 cells treated with GM-CSF at concentrations of 1ng/ml and 0.1ng/ml. A linear trend exists over time when cells were treated with 0.1ng/ml (R2 = 0.99).
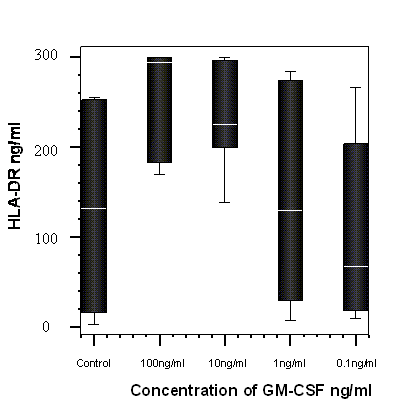
The percentage change from control of soluble HLA-DR detected in cells cultured with GM-CSF and in media alone (control) after 8 hours incubation with GM-CSF (n=7). A significant dose dependant trend was observed, p = 0.04, Friedman test.
Author biography
Sara Perry obtained her BSc in Microbiology and Immunology from University of Leeds, and PhD in Immunology from University of Liverpool, where she worked on the expression of HLA-DR and the characterisation of the monocyte in patients with sepsis. She is currently a Research Fellow at University of Leeds working on Immunotherepy for colorectal cancer.
Sobhy Mostafa, MB, ChB (Cairo, 1966), MRCS (Eng, 1976), LRCP (Lond, 1976), FRCA (Eng 1973), MD (Liverpool 2000), is a consultant of Anaesthesia & Intensive Care Medicine at Royal Liverpool University Hospital. Research interests include: Tumour necrosis factor in patients with acute respiratory distress syndrome, Local production of inflammatory cytokines and plasma exchange, sepsis and survival in ICU patients.
Richard Wenstone, M.B.,Ch.B.(Manc. 1983), D.A. (UK 1985), F.F.A.R.C.S. (Eng. 1988), is currently a consultant in Anaesthesia and Intensive Care at the Royal Liverpool and Broadgreen University Hospitals. His current research activities include the relevance of HLA-DR and GM-CSF in septic patients. This work, in co-operation with the University of Liverpool Departments of Immunology and Clinical Chemistry, has resulted in a number of publications. It is shown that neither monocyte HLA-DR, although frequently depressed in sepsis, nor plasma levels of soluble HLA-DR, correlate with prognosis in those patients. Both of these measurements are increased by GM-CSF and low levels of this are associated with a very poor prognosis
Alan Shenkin is a Professor of Clinical Chemistry at the University of Liverpool. He holds consultant status in the Royal Liverpool University Hospital with responsibility for the NHS diagnostic laboratories in clinical biochemistry. He has a special interest in the hospital for nutritional care of very sick patients.
Paul J McLaughlin obtained the BSc in Medical microbiology at Liverpool University and PhD in tumour immunology at Nottingham University, and is currently a senior lecturer in Immunology with research interest in HLA-DR Expression in Sepsis.
![]() Corresponding address:
Corresponding address:
Paul J McLaughlin, Department of Immunology, University of Liverpool, Duncan Building, Daulby Street, Liverpool, L69 3GA. Email: bmbsepleeds.ac.uk, Tel No: 0113 3433130.

 Global reach, higher impact
Global reach, higher impact