Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(2):116-125. doi:10.7150/ijms.1.116 This issue Cite
Research paper
A Randomized Study of Epithelial Ovarian Cancer: Is Chemotherapy Useful after Complete Remission?
GOCCNE Group (Gruppo Oncologico Cooperativo Clinico Nord-Est ovaio), Padua, Italy
Received 2004-3-23; Accepted 2004-5-17; Published 2004-6-1
Abstract
Objective. The aim of this study is to verify whether consolidation chemotherapy with Cisplatin improves disease-free survival and/or overall survival in patients affected by epithelial ovarian cancer.
Methods. A multicenter study examined 122 randomized patients in complete remission as judged by laparoscopy or laparotomy following first-line chemotherapy consisting of ACy (Adriamycin + Cyclophosphamide), PCy (Cisplatin + Cyclophosphamide), or Mitoxantrone + Carboplatin. Sixty-one of these patients were treated with 3 cycles of 5-Fluorouracil (FU) 500 mg/m2 for 5 days followed by Cisplatin at 100 mg/m2 on the 6th or 7th day every 28 days; the other 61 received no further treatment (nihil group).
Results. Sixty patients in the Cisplatin arm were evaluable. There were 36 relapses in the FU+Cisplatin arm and 30 in the nihil arm. Peritoneal relapses were 25% for Cisplatin treatment vs. 16.4 % for nihil. There were 29 deaths in the Cisplatin arm vs. 27 for nihil. Median overall survival time (95 months with Cisplatin vs. 96 months in the nihil group) and median disease-free survival (66 months with Cisplatin vs. 73 in the nihil group) were similar in both arms (p=0.66 and p=0.41, respectively). There were no significant differences in tumor stage and grade between the two arms. Seven patients presented a second neoplasm during follow-up: six in the nihil arm, but only one patient in the Cisplatin arm. Death in these patients was due to the second neoplasm and not to progression of ovarian cancer.
Conclusion. Three courses of additional platinum+FU treatment after five cycles of first-line chemotherapy without FU produced no increase in overall survival or disease-free survival.
Keywords: Epithelial Ovarian Cancer, Consolidation Chemotherapy, Cisplatin, Fluorouracil, Absence of Residuum
1. Introduction
Cure rates for advanced ovarian cancer are low, regardless of the type of treatment received. An average 5-year survival rate of 10% (0-15%) has been obtained with abdominal radiation therapy [1-4], and response rates vary from 3 to 30% with chemotherapy [5-9]. Recent multivariate analyses of prognostic factors suggest that the size of the residual tumor after first surgery is the most important variable in predicting the response to therapy [3]; for each 10% increase in maximum cytoreduction, survival increased by 5.5%. There was an average difference in survival of approximately 11 months between the less-than-25% and the greater-than-75% maximum cytoreduction cohorts [10].
The use of Cisplatin, which presents encouraging response rates, is associated with longer survival [11]; however, often the efficacy of highly aggressive regimens is only seen in those patients with highly favorable prognostic factors (optimal cytoreductive surgery). According to the Goldie-Coldman theory, early administration of aggressive regimens could selectively interfere with the number of drug-resistant cells arising by spontaneous mutation, reducing them to a minimum [1] and therefore possibly neutralizing the risk of relapse. The relapse rate for epithelial ovarian carcinoma in pathologic Complete Remission is generally between 42 and 44% [12-14]. Abdominal radiotherapy as consolidation treatment produced forms of gastrointestinal toxicity, such as subocclusion and enteritis, without significant therapeutic advantage [15-17].
In early ovarian cancer, adjuvant chemotherapy is effective [18]; Trimbos et. al. reported results from a randomized trial, one conducted by the EORTC (European Organisation for Research and Treatment of Cancer) (ACTION Study) where immediate chemotherapy significantly improved recurrence-free survival with an absolute difference at five years of 8%, but with a greater advantage found among patients having had non-optimal staging or surgical treatment at inexperienced centers.
However, other randomized studies have shown that disease-free survival and overall survival in advanced ovarian cancer do not vary in relation to the number of cycles of chemotherapy given, (6 vs. 9 cycles of PAC [Platinum-Adriamycin-Cyclophosphamide] [19], 5 vs. 10 cycles of polychemotherapy with Cisplatin [20], 6 vs. 12 cycles of PAC [21] or 5 vs. 8 courses of platinum [22]), while still other trials on a small number of patients with absent disease or residuum <2cm (table 1) would seem to confirm the usefulness of radiotherapy or of chemoradiotherapy as consolidation [23].
2. Patients and Methods
From October 1988 to October 1996, 122 patients affected by epithelial ovarian cancer who had been found to be in complete remission, as judged by laparoscopy and/or laparotomy, entered the GOCCNE study (Gruppo Oncologico Cooperativo Clinico del Nord-Est, consisting of 20 centers in North-east Italy) designed to evaluate the usefulness of consolidation chemotherapy in patients with pathologic complete remission. First-line chemotherapy consisted of Anthracyclin-Cyclophosphamide for 54 patients, Mitoxantrone-Carboplatin for 5 elderly patients and Cyclophosphamide-Platinum for 63 patients. Pathologic complete remission was assessed by CT scan in all patients; if this was negative, the same patients underwent laparoscopic control. All patients with negative laparotomy (70 pts; however, 1 pt. after a negative laparoscopy, at time of randomization resulted positive at second look laparotomy performed autonomously) and/or negative laparoscopic second looks (52 pts) were proposed for the study and randomly assigned to consolidation chemotherapy with 5-Fluorouracil + Cisplatin or nihil.
Inclusion in the study was based on a histologically documented diagnosis, completion of first-line chemotherapy and a performance status higher than 60. In addition, it was required that patients have normal renal function (creatinine <2 mg/dL, BUN <45 mmol/L), hepatic function (conjugated bilirubin <2 mg/dL, ALP <150 U/L and γGT <80 U/L) and cardiac function (as judged by physical examination, measurement of pulse and arterial pressure and ECG). Patients were also required to give their informed consent.
Patients over 80 years of age were excluded, as were patients with WBC counts <4000/mm3 or platelets <140,000/mm3. Patients presenting with second tumors, psychiatric disorders, brain metastases and those with altered ECGs, suggesting important conduction disturbances (dromotropic or bathmotropic), were also excluded.
Consolidation chemotherapy with 5-Fluorouracil (5-FU) and Cisplatin consisted of 5-FU 500 mg/m2/day intravenously for 5 days, followed by Cisplatin at a dose of 100 mg/m2 on the 6th or 7th day (never exceeding a total dose of 160 mg) associated with Allopurinol 300 mg/day. This was repeated every 28 days for 3 cycles. The choice of 5-Fluorouracil was based on the fact that 5-FU had been shown to be highly effective in salvage therapy for refractory ovarian cancer patients with low bulk disease [24, 25].
In cases developing arterial hypertension or myocardiosclerosis (with diastolic pressure >120mmHg) during treatment, the dose of 5-FU was reduced by 25%. The first day of 5-FU was abolished in cases of myelotoxicity protracted from the previous cycle (more than 5 weeks to recovery). Cisplatin was reduced by 50% in mononephric patients, or when obstructive nephropathy was encountered, as well as in cases of important hypacusia or neurotoxicity or in patients over 76 years of age.
Statistical comparison of overall and disease-free survival was based on Kaplan-Meier estimates of survival and a log-rank test of statistical significance.
3. Results
The study population consisted of 122 patients. Median age of both the consolidation therapy population and the nihil arm was 55 (range: 38-76 and 16-73, respectively). The stage distribution was 15 pts stage I C, 30 pts stage II B and II C, 71 pts stage III, and 5 pts stage IV; histologic grade was grade 1 in 19 cases, 2 in 45 cases and 3 in 57 cases. Distribution of histological grade for the Cisplatin arm vs. the nihil arm was grade 1-2 in 30 vs. 34 cases and grade 3 in 30 vs. 27 cases. Characteristics of the two groups can be found in table 2.
The 61 patients in the nihil arm completed 5 cycles of first-line chemotherapy only, while an additional 3 cycles of polychemotherapy with FU followed by Cisplatin were proposed for the 61 patients in the Cisplatin arm. Of the 61 patients assigned to the Cisplatin arm, 47 (77.0%) completed 3 or more cycles. However, 4 patients (6.6%) completed only 1 cycle while 9 patients (14.8%) completed 2 cycles before suspending treatment (for noncompliance or for excessive gastrointestinal and/or neurological toxicity). One patient (1.6%) refused treatment entirely after randomization and is therefore not evaluable. Three of the 47 patients who completed 3 cycles continued treatment even further, receiving an additional 1-3 cycles of Cisplatin for a total of 4-6 cycles (table 2).
The toxicity in treatment with Cisplatin consisted of nausea and vomiting in 38 patients (62.3% of Cisplatin-treated patients); this was WHO grade 3 in 17 pts (27.9%) and grade 4 in 10 pts (16.4%); it was neurological in 21 pts (34.4%), and consisted of leukopenia in 13 pts (21.3%), mucositis in 7 pts (11.5%), nephrotoxicity in 4 pts (6.6%), infection in 3 (4.9%) and anemia in another 2 (3.3%) pts (table 3).
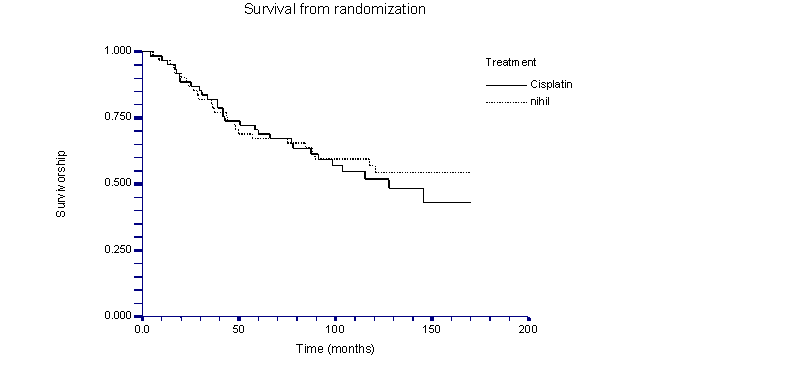
The overall survival of the two groups was very similar (fig.1.). There were 36 relapses (59.0%) in the Cisplatin arm and 30 (49.2%) in the “nihil” arm. Peritoneal relapses varied for the two arms and were 15 (25%) for Cisplatin vs. 10 (16.4%) for nihil. Relapses in the vaginal dome were found in 1 case (1.7%) treated with Cisplatin and 5 (8.2%) in the nihil arm, while hepatic and splenic relapses were nearly identical. Relapses in lung-pleura were also similar: 5 (8.3%) in the Cisplatin arm vs. 6 (9.8%) in the nihil arm. Relapses in lumboaortic-inguinal lymph nodes were 11 (18.0%) in the Cisplatin arm and 4 (6.6%) in the nihil arm (table 4).
Seven patients presented a second neoplasm after treatment for ovarian cancer. There were two cases of breast cancer, two cases of sigmoid adenocarcinoma, one case of small cell lung cancer, one case of hypernephroma, and one case of head and neck cancer. Six of these patients were in the nihil arm. Only one patient (head and neck cancer) was in the Cisplatin arm. Death in these patients resulted from the second neoplasm and not from progression of ovarian cancer.
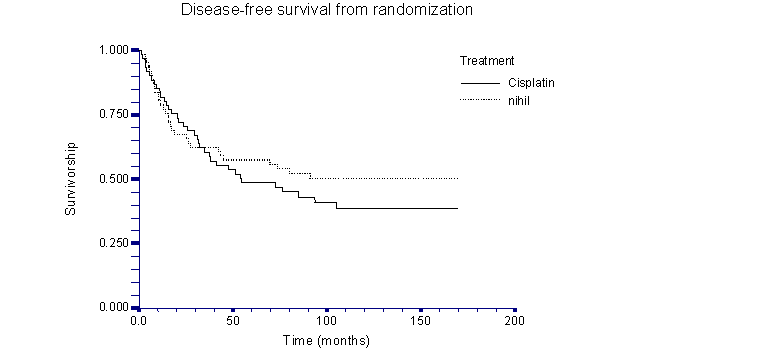
Median overall survival was 87 months for the cisplatin arm (82.0% at 3 years) and 89 months for the nihil arm (80.3% at 3 years) (p=0.66, log rank test). Median disease free survival was 68 months (range 1.4-170.0) for the Cisplatin arm (62.1% at three years for the cisplatin arm) and 73 months (range 1.6-169.5) for the nihil arm (62.3% at three years for the nihil arm). There was no statistical difference between the two groups (p=0.41, log rank test).
4. Discussion
Ovarian cancer patients have high response rates to initial chemotherapy after cytoreductive surgery. There are fewer complete responses with the use of anthracyclin and alkylating agents as first-line treatment, although their duration is greater than the complete responses obtained by platinum chemotherapy [5]. Relapses occur in 30% of the cases within the first 3 years [26]. It is thus possible that the use of alkylating agents and anthracyclins could select responders who have a different prognosis from patients treated with a platinum-based regime. Unlike Gershenson [27] who sustains the usefulness of prolonged chemotherapy with platinum, other investigators have found that prolonging chemotherapy does not have any benefit in terms of survival [19-22].
In the literature there is no agreement regarding treatment of patients with ovarian cancer who are in complete remission following first-line chemotherapy, and no therapeutic modality shows a clear and definite advantage in terms of disease-free survival. Moreover, there are no large comparative randomized studies on this topic [19-21, 26].
In 1988 when the present trial was proposed there was uncertainty as to whether 3 additional courses of platinum based chemotherapy were useful in patients who had obtained complete remission after 5 courses of first-line chemotherapy with or without Cisplatin. The choice of Cisplatin + 5-FU was based on the fact that other drugs such liposomal Doxorubicin, Paclitaxel, or Topotecan were not available at that time.
Radiation therapy was not chosen for consolidation treatment because the literature provided no studies to support a significant advantage over chemotherapy [26, 28, 29] while presenting local toxicity. Still, Pickle et al. [23], in a randomized study, demonstrated the usefulness of radiation therapy in association with chemotherapy in patients (predominantly stage III) who were judged free of disease, after radical surgery and six cycles of first-line chemotherapy, with respect to thirty-two patients who received only whole abdominal radiation following surgery. The overall and relapse-free survival was better for the group receiving combination chemo-radiotherapy, confirming the fundamental role of systemic treatment.
Our experience with only 121 evaluable patients, however, suggests that there is no difference between chemotherapeutic consolidation after remission vs. nihil. This trial therefore seems to confirm the findings of other authors, namely that the 5-year-survival of patients without residual tumor mass following first surgery varies between 50 and 70% [3, 30], and that their survival is better than that of patients with residual mass <2 cm (which varies between 35-55%) or patients with residuum of 2 cm or more (which varies between 0-30%) [3, 7].
While statistical analysis of the present study does not note any significant difference in overall survival (fig. 1) or in disease-free survival (fig. 2) between the two arms suggesting that polychemotherapy with cisplatin is not very effective in a consolidative setting, it is limited by the lack of stratification by stage, and by previous type of chemotherapy (table 2) as well as by the limited number of patients randomized (122 pts). The lack of stratification might have resulted in the selection of a population with better prognostic factors in the nihil arm vs. the Cisplatin arm, thereby creating a bias; thus, this result should be evaluated with caution
It is worth noting that many of our patients had not been previously treated with platinum, and yet survival rates were similar to those for patients with platinum based first-line therapy. This would seem to cast doubts on the long-term efficacy of platinum therapy in ovarian cancer.
In our study severe toxicity was rare with the exception of grade 3-4 emesis encountered in 44,3%. Severe nausea was controlled in most of the patients; however, in 21.3% of patients toxicity was severe enough to suspend treatment either for non-compliance or for excessive gastrointestinal toxicity, thus cycles were suspended in 13 patients (4 after completing only one cycle, while 9 refused further treatment after completing the second cycle). Our patients did not experience severe neuropathy. In addition, severe events such as sepsis or hematologic toxicity were also uncommon in our study with the exception of 1 case of grade 4 thrombocytopenia.
Oddly, there were more relapses in lumboaortic-inguinal lymph nodes in the chemotherapy arm, however, in this study the different distribution of patients with regard to residuum and stage, which resulted from a lack of stratification, might have produced a lack of benefit in terms of recurrence and survival.
Currently, tumor cell heterogeneity and clonal selection of resistant tumor cells continue to be major obstacles in cancer therapy. In vitro experimental studies are limited in that they cannot represent the heterogeneity of cancer cells observed in vivo. On the other hand, some immunohistochemical correlation with clinical aspects of disease is starting to emerge [31, 32, 33, 34] and may eventually provide clues for further tailoring of therapy to the specific patient. In addition, in programming new therapeutic approaches it is necessary to understand the percentage of relapses after complete remission that are due to residual tumor cells and how many might be due to a perpetuation of carcinogenesis in the peritoneal cavity. To date, the most important variable in survival of patients with epithelial ovarian cancer is residual disease > 10 cm after first surgery; these patients present with an extremely high relapse rate. Thus, “wait and see” is still the main choice for patients who have reached complete remission after first-line chemotherapy in the absence of confirmatory data from randomized trials.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Fuks Z. External radiotherapy of ovarian cancer: Standard approaches and new frontiers. Semin Oncol. 1975 ;2 :253 -266
2. Dembo AJ. Radiotherapeutic management of ovarian cancer. Semin Oncol. 1984 ;11 :238 -250
3. Martinez A, Schray MF, Howes AE. Postoperative radiation therapy for epithelial ovarian cancer: the curative role based on a 24-years experience. J Clin Oncol. 1985 ;3 :901 -922
4. Fuks Z, Yahalom Y, Brenner H. The treatment of ovarian carcinoma. In: (ed.) Nori D, Hilaris BS. Radiation Therapy of Gynecological Cancer. New York: Liss. 1987 :147-172
5. Wiltshaw E, Evans B, Rustin G. A prospective randomized trial comparing high-dose cisplatin with low-dose cisplatin and chlorambucil in advanced ovarian carcinoma. J Clin Oncol. 1986 ;4 :722 -729
6. Parker LM, Griffiths CT, Yankee RA. Combination chemotherapy with Adriamycin-cyclophosphamide of advanced ovarian carcinoma. Cancer. 1980 ;46 :669 -674
7. Wharton JT, Edwards CL, Rutledge FN. Long term survival after chemotherapy for advanced epithelial ovarian carcinoma. Am J Obstet Gynecol. 1984 ;148 :997 -1005
8. Williams CJ, Mead GM, Macbeth RF. Cisplatin combination chemotherapy versus chlorambucil in advanced ovarian carcinoma: mature results of randomized study. J Clin Oncol. 1985 ;3 :1455 -1462
9. Louie KG, Ozols RF, Myers CE. Long-term results of a cisplatin-containing combination chemotherapy regimen for the treatment of advanced ovarian carcinoma. J Clin Oncol. 1986 ;4 :1579 -1585
10. Bristow RS, Tomacruz DK, Armstrong EL. et al. Survival impact of maximum cytoreductive surgery for advanced ovarian carcinoma during the platinum-era: a meta-analysis of 6,848 patients. Proc ASCO. 2001 ;20 (1) :202a -
11. Greco FA, Hande KR, Jones HW. et al. Advanced ovarian cancer: long-term follow up after brief intensive chemotherapy. Proc ASCO. 1984 ;3 :166 -
12. Rubin SC, Hoskins WJ, Saigo PE. et al. Prognostic factors for recurrence following negative second-look laparotomy in ovarian cancer patients with platinum-based chemotherapy. Gynecol Oncol. 1991 ;42 (2) :137 -141
13. Neijt JP. Treatment of advanced ovarian cancer: 10 years of experience. Ann Oncol. 1992 ;3 :17 -27
14. Luesley D, Blackledge G, Kelly K. et al. Failure of second-look laparotomy to influence survival in epithelial ovarian cancer. The Lancet. 1988 ;10 :599 -603
15. Hainsworth JD, Malcom A, Johnson DH. et al. Advanced minimal residual ovarian carcinoma: abdominopelvic irradiation following combination chemotherapy. Obstet Gynecol. 1983 ;61 (5) :619 -623
16. Fuks Z, Rizel S, Biran S. Chemotherapeutic and surgical induction of pathological complete remission and whole abdominal irradiation for consolidation does not enhance the cure of stage III ovarian carcinoma. J Clin Oncol. 1988 ;6 :509 -516
17. Hoffman MS, Greenberg H, Finan M. et al. Whole-abdomen radiation as a second-line therapy for epithelial ovarian cancer. Gynecol Oncol. 1989 ;35 (1) :73 -74
18. Trimbos BJ, Vergote I, Bolis G. et al. Impact of Adjuvant Chemotherapy and Surgical Staging in Early-Stage Ovarian Carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm Trial. J Natl Cancer Inst. 2003 ;95 (2) :113 -125
19. Watring W, Semrad N, Alverdian V. et al. Second-look procedures in ovarian cancer patients receiving six vs. nine courses of platinum, adriamycin, cytoxan (PAC) chemotherapy: the SCPMG experience 1982-1985. Gynecol Oncol. 1989 ;32 (2) :245 -247
20. Hakes TB, Chalas E, Hoskins WJ. et al. Randomized prospective trial of 5 versus 10 cycles of cyclophosphamide, doxorubicin and cisplatin in advanced ovarian carcinoma. Gynecol Oncol. 1992 ;45 (3) :284 -289
21. Bertelsen K, Jakobsen A, Stroyer I. et al. A prospective randomized comparison of 6 and 12 cycles of Cyclophosphamide, Adriamycin and Cisplatin in advanced epithelial ovarian cancer: a Danish ovarian study Group Trial (DACOVA). Gynecol Oncol. 1993 ;49 :30 -36
22. Lambert HE, Rustin GJS, Gregory WM. et al. A randomized trial of five versus eight courses of cisplatin or carboplatin advanced ovarian carcinoma: a North Themes Ovary Group study. Ann Oncol. 1997 ;8 :327 -33
23. Pickel H, Lahousen M, Petru E. et al. Consolidation radiotherapy after carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol. 1999 ;72 :215 -219
24. Doroshow J, Braly P, Hoff S. et al. Intraperitoneal chemotherapy with cisplatin and 5-fluorouracil: an active regimen for refractory ovarian cancer. Proc ASCO. 1986 ;5 :117 -
25. Fiorentino MV, Nicoletto MO, Smergo A. et al. 5-Fluorouracil and platin as second line in ovarian cancer. Second International Symposium on “Multimodality of treatment in Ovarian Cancer”. 1987, September 24-26 Genoa, Italy
26. Goldhirsch A, Greiner R, Dreher E. et al. Treatment of advanced ovarian cancer with surgery, chemotherapy, and consolidation of response by whole-abdominal radiotherapy. Cancer. 1988 ;62 :40 -47
27. Gershenson DM, Mitchell MF, Atkinson N. et al. The effect of prolonged cisplatin-based chemotherapy on progression-free survival in patients with optimal epithelial ovarian cancer: “maintenance” therapy reconsidered. Gynecol Oncol. 1992 ;47 :7 -13
28. Menczer J, Ben-Baruch G, Modan M. et al. Intraperitoneal cisplatin chemotherapy versus abdominopelvic irradiation in ovarian carcinoma patients after second-look laparotomy. Cancer. 1989 ;63 :1509 -1513
29. Menczer J, Ben-Baruch G, Rizel S. et al. Intraperitoneal chemotherapy with cisplatin and etoposide in ovarian carcinoma patients who are clinically in complete remission. Eur J Gynaecol Oncol. 1995 ;16 (1) :12 -17
30. Dembo AJ. Abdominopelvic radiotherapy in ovarian cancer. A 10-years experience. Cancer. 1985 ;55 :2285 -2290
31. Baekelandt MM, Holm R, Nesland JM. et al. P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer Res. 2000 ;20 (2B) :1061 -1067
32. Xiang J, Gomez-Navarro J, Arafat W. et al. Pro-apoptotis treatment with adenovirus encoding Bax enhances the effect of chemotherapy in ovarian cancer. J Gene Med. 2000 ;2 (2) :97 -106
33. Petty R, Evans A, Duncan I. et al. Drug resistance in ovarian cancer - the role of p53. Pathol Oncol Res. 1998 ;4 (2) :97 -102
34. Skirnisdottir I, Seidal T, Gerdin E, Soreb B. The prognostic importance of p53, bcl-2, and bax in early stage epithelial ovarian carcinoma treated with adjuvant chemotherapy. Int J Gynecol Cancer. 2002 ;12 :265 -276
35. Falcone A, Chiara S, Franzone P. et al. Moving-strip abdomino-pelvic radiotherapy after cis-platinum-based chemotherapy and second-look operation. A feasibility study in advanced ovarian cancer. Am J Clin Oncol. 1988 ;11 (1) :16 -20
36. Nicoletto MO, Fiorentino M, Vinante O. et al. Experience with intraperitoneal alpha-2a interferon. Oncology. 1992 ;49 :467 -473
37. Barakat RR, Almadrones L, Venkatraman ES. et al. A phase II trial of intraperitoneal cisplatin and etoposide as consolidation therapy in patients with stage II-IV epithelial ovarian cancer following negative surgical assessment. Gynecol Oncol. 1998 ;69 :17 -22
Tables and Figures
Studies of efficacy of consolidation treatment in ovarian cancer
| Study | Year | N. pts. | Stage | Treatment | Residuum before consolidation | Efficacy | |
| 1. | Falcone [35] | 1988 | 16 | IIB-IV | WAR | Microscopic disease (10 pts) | Slight advantage for microscopic disease |
| < 2 cm (6 pts) | |||||||
| 2. | Nicoletto [36] | 1992 | 18 | IIIA-B | i.p. αIFN x 4 to CCR + 4 cycles consolidation | Microscopic disease (11 pts) | Possibly effective in microscopic disease |
| < 2 cm (7 pts) | |||||||
| 3. | Menczer [28, 29] | 1989 | 37 | II-IV | i.p. cisplatin x 3 | i.p. cisplatin might be better than RT | |
| WAR | |||||||
| 1995 | 21 | i.p. cisplatin | CCR 17 SL (10+; 7-) | i.p. cisplatin+etoposide does not seem more effective than i.p. cisplatin alone | |||
| 4. | Bruzzone [33] | 1997 | 111 | JM8 400 i.p. | αIFN does not improve D.F. or O.S. | ||
| αIFN+JM8 400 i.p. | |||||||
| 5. | Barakat [37] | 1998 | 36 | IIC-IV | i.p. cisplatin VP 16 x 3 | Microscopic disease (13 pts) | i.p. cisplatin improved disease-free survival |
| Nihil | |||||||
| 6. | Pickel [23] | 1999 | 64 | 32 pts - WAR | RT offers promising results | ||
| 32 pts - nihil | |||||||
WAR= whole abdominal radiation; RT = radiotherapy; nihil = no treatment; D.F.= median disease-free survival; O.S.= median overall survival; i.p. = intraperitoneal; CCR= complete clinical remission; JM8 = Carboplatin; αIFN = interferon α; SL = second look
Patient characteristics
| Characteristics | Cisplatin (n=60 pts) | Nihil (n=61 pts) | |
| 1. | Median Age (years) | 55 | 55 |
| Range | 38-76 | 16-73 | |
| 2. | Stage (FIGO) | ||
| IC | 3 (4.9%) | 12 (19.7%) | |
| IIB-C | 13 (21.3%) | 17 (27.9%) | |
| IIIA-B-C + IV | 45 (73.8%) | 32 (52.4%) | |
| 3. | Histological Type | ||
| Serous Papillary | 45 (73.8%) | 32 (52.5%) | |
| Undifferentiated | 3 (4.9%) | 6 (9.8%) | |
| Clear Cell | 2 (3.3%) | 2 (3.3%) | |
| Endometrioid | 8 (13.1%) | 15 (24.6%) | |
| Mucinous | - | 4 (6.6%) | |
| Mixed | 3 (4.9%) | 2 (3.3%) | |
| 4. | Histological Grade | ||
| 1-2 | 30 (49.2%) | 34 (55.7%) | |
| 3 | 31 (50.8%) | 27 (44.3%) | |
| 5. | Type of first line chemotherapy | ||
| Anthracyclin-Cyclophosphamide | 30 (49.2%) | 24 (39.3%) | |
| Mitoxantrone-Cisplatin | 2 (3.3%) | 3 (4.9%) | |
| Cyclophosphamide-Cisplatin | 29 (47.5%) | 34 (55.7%) | |
| 6. | Type of Surgery | ||
| Laparoscopy | - | 1 (1.6%) | |
| Tumor Reduction | 13 (21.3%) | 5 (8.2%) | |
| Radical Surgery | 48 (78.7%) | 55 (90.2%) | |
| 7. | Postsurgical Residual Disease (first look) | ||
| Residuum ≤2 cm | 51 (83.6%) | 55 (90.2%) | |
| Residuum >2 cm | 10 (16.4%) | 6 (9.8%) | |
| 8. | Total number of cycles completed | ||
| 0 | 1 (%) | - | |
| 1 | 4 (%) | - | |
| 2 | 9 (%) | - | |
| 3 | 44 (%) | - | |
| 4 | 2 (%) | - | |
| 5 | - | - | |
| 6 | 1 (%) | - | |
Toxicity in patients who received Cisplatin consolidation therapy
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total | |
| 1. | Allergic reaction | - | - | 1 (1.6%) | - | 1 (1.6%) |
| 2. | Alopecia | - | 4 (6.6%) | 1 (1.6%) | - | 5 (8.2%) |
| 3. | Hematologic | 4 (6.6%) | 11 (18.0%) | 4 (6.6%) | - | 19 (31.1%) |
| - Anemia | 2 (3.3%) | - | - | - | 2 (3.3%) | |
| - Leukopenia | 3 (4.9%) | 10 (16.4%) | - | - | 13 (21.3%) | |
| - Neutropenia | 2 (3.3%) | - | 1 (1.6%) | - | 3 (4.9%) | |
| - Thrombocytopenia | 1 (1.6%) | 1 (1.6%) | - | 1 (1.6%) | 3 (4.9%) | |
| 4. | Infection | 2 (3.3%) | - | 1 (1.6%) | - | 3 (4.9%) |
| 5. | Mucositis | 3 (4.9%) | 3 (4.9%) | - | 1 (1.6%) | 7 (11.5%) |
| 6. | Nausea-Vomiting | 1 (1.6%) | 10 (16.4%) | 17 (27.9%) | 10 (16.4%) | 38 (62.3%) |
| 7. | Renal | 4 (6.6%) | - | - | - | 4 (6.6%) |
Relapses: isolated or multiple relapses divided by site.
| Site of relapse | Cisplatin (n=61 pts) | Nihil (n=61pts) | |
| Total | 36 (59.0%) | 30 (49.2%) | |
| 1. | Peritoneum | 15 (24.6%) | 10 (16.4%) |
| 2. | Retroperitoneum | 3 (4.9%) | 2 (3.3%) |
| 3. | Pelvis | 5 (8.2%) | 11 (18%) |
| 4. | Lumbo-aortic LN | 6 (9.8%) | 3 (4.9%) |
| 5. | Inguinal LN | 5 (8.2%) | 1 (1.6%) |
| 6. | Supraclavear LN | 2 (3.3%) | 1 (1.6%) |
| 7. | Vaginal dome | 1 (1.6%) | 5 (8.2%) |
| 8. | Spleen | 1 (1.6%) | 2 (3.3%) |
| 9. | Lung-pleura | 5 (8.2%) | 6 (9.8%) |
| 10. | Liver | 3 (4.9%) | 4 (6.6%) |
| 11. | CA 125 | 5 (8.2%) | 1 (1.6%) |
| 12. | Bone | 1 (1.6%) | 1 (1.6%) |
| 13. | Rectum-sigma | 1 (1.6%) | 1 (1.6%) |
| 14. | Ileo-paraintestinal | 1 (1.6%) | 3 (4.9%) |
| 15. | Peritoneum + Pelvis+ Vaginal dome + Rectum-sigma + Ileo-paraintestinal | 23 (37.7%) | 30 (49.2%) |
| 16. | Retroperitoneum + Lumbo-aortic LN + Inguinal LN + Supraclavear LN | 16 (26.2%) | 7 (11.5%) |
LN = lymph node
Kaplan-Meier curve of survival from time of randomization.
Kaplan-Meier curve of disease-free survival from time of randomization.
Author biography
M. O. Nicoletto is the Associate Medical Director of the Division of Medical Oncology at the Civil Hospital of Padua, Italy. At present, her duties include teaching in the Oncology Residency program. She is the coordinator of the Gruppo Oncologico-Clinico-Nord-Est-Ovarian-Cancer (GOCNE). She is also responsible for the local screening group for hereditary tumors, and cooperates with national and international ICON studies. Her primary research interests are ovarian cancer and hereditary breast-ovarian neoplasms.
S. Tumolo is currently the Chief of the Oncology Unit at “Santa Maria degli Angeli” Hospital in Pordenone, Italy. His past positions include full-time associate at the Division of Radiotherapy and Medical Oncology, Ospedale Civile in Pordenone, for 6 years and at the Division of Medical Oncology, Centro di Riferimento Oncologico, in Aviano, for 13 years. He is actively involved in various areas of research, in the past as a member of the Gynaecological Cancer Group of the European Organization for Research and Treatment of Cancer (EORTC).
C. Falci recently finished her Medical Degree at the University of Padua with a thesis in collaboration with the Mario Negri Institute in Milan and continues to cooperate with the ICON studies.
M. Donach, having completed his B.A. in Biochemistry at New York University, is presently in his final year of medical studies at the University of Padua and will pursue resident training in Oncology. His current interests include cancer cell biology and research in the field of ovarian cancer.
![]() Corresponding address:
Corresponding address:
M. O. Nicoletto, Department of Medical Oncology, Ospedale Busonera, via Gattamelata 64, 35128 Padova, Italy. Tel: ++39 049 821-5927, fax: ++39 049 821-5928, e-mail: mariaornit; mariaornella.nicolettoit

 Global reach, higher impact
Global reach, higher impact