3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2004; 1(1):43-49. doi:10.7150/ijms.1.43 This issue Cite
Case report
Cribriform-Morular Variant of Papillary Carcinoma: Association with Familial Adenomatous Polyposis - Report of Three Cases and Review of Literature
Department of Pathology, University of Texas Health Science Center at San Antonio, San Antonio, Texas 78229-3900, USA
Received 2004-2-6; Accepted 2004-3-17; Published 2004-3-20
Abstract
We describe a rare variant of papillary thyroid carcinoma (PTC), the Cribriform-Morular Variant (C-MV). A handful of cases have been described in the literature of this entity. They exhibit the morphologic features of a distinctive papillary neoplasm along with solid, cribriform, and squamoid-morular areas. The cribriform and morular features make this a separate entity which could be mistaken for a high grade aggressive thyroid neoplasm. These lesions are usually associated with familial adenomatosis polyposis (FAP) but rarely may be sporadic. We report three cases that we have encountered.
Keywords: thyroid neoplasm, papillary thyroid carcinoma, cribriform, squamoid, morular
1. Report of cases
Case 1
Patient was a 32 year old G1P0 white female who presented with an enlarging painless lump in her neck for the preceding two years. Ultrasound revealed a large, lobulated, right lobe mass. Serum level showed: TSH-0.4 μ IU/ml, T3-1.88ng/ml (N: 0.6-1.81 ng/ml), free T4-1.2 ng/dl (N: 0.8-1.8 ng/dl) and 24 hour radio active iodine uptake-37%. She underwent a total thyroidectomy and is currently on adjuvant radioactive iodine. She also underwent a proctocolectomy for multiple colonic polyps and colonic carcinoma.
Case 2
Patient was a 14 year old Latin American female with a gradually enlarging right neck mass for 2 years. CT scan showed several bilateral lesions. Fine needle aspiration showed normal follicular cytology. Serum level showed: TSH-1.31 μ IU/ml, T4-0.92ng/ml (N: 0.6-1.81 ng/ml), free T4-2.6 ng/dl (N: 0.8-1.8 ng/dl) and 24 hour radio active iodine uptake-28%. She underwent a total thyroidectomy with subsequent radioactive ablation. There is no family history of colonic polyposis.
Case 3
Patient was a 34 year old asymptomatic HIV positive female with bilateral neck masses. Fine needle aspiration of the left lobe lesion showed papillary carcinoma. Serum level showed: TSH-1.92 μ IU/ml, free T4-1.1 ng/dl (N: 0.8-1.8 ng/dl) and 24 hour radio active iodine uptake-31%. She underwent a total thyroidectomy with subsequent radioactive ablation. She had a colectomy for multiple colonic polyps.
Gross Pathology
Specimens revealed well-circumscribed, somewhat lobulated tan masses ranging from 1.5-2 cm with multiple satellite nodules. There was no lobe predilection. There was no necrosis or hemorrhage seen. The remainder of the thyroid was lobulated and beefy. Few lymph nodes were also identified.
Microscopic Pathology
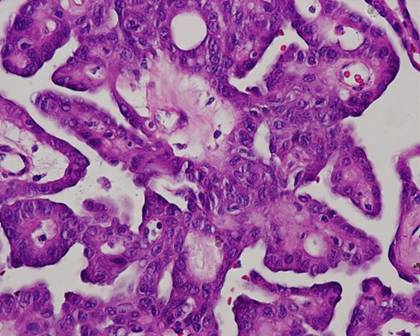
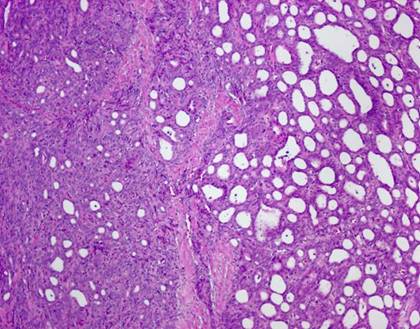
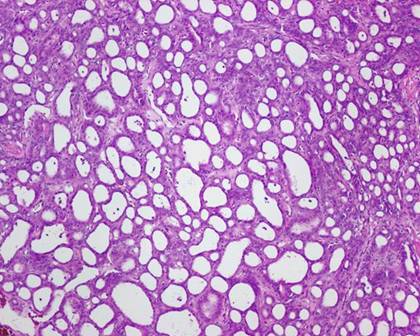
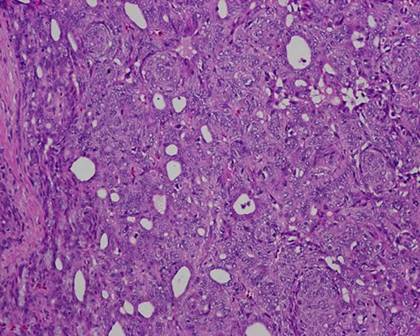
The typical nuclear features of PTC could be seen. They were complex branching papillary structures lined by cuboidal cells. The nuclei were hyperchromatic, optically clear with longitudinal grooves and showed eosinophilic, intranuclear and cytoplasmic inclusions as in classic PTC (Fig 1). Some lesions showed an intricate blending of several histological patterns (Fig 2). Cribriform areas had back-to-back follicles with anastomozing bars and arches of cells in the absence of intervening fibrovascular stroma and accounted for 30-50% of the lesions (Fig 3). The solid areas, which were approximately 20-30%, consisted of whorls of cells that form squamoid morules or nests that typically do not show any keratinization or intercellular bridges (Fig 4). No colloid or psamomma bodies were found in the tumors. Case 3 also showed foci of tall and columnar cell features.
2. Comment
Cribriform-Morular Variant (C-MV) of PTC is a rare morphologic entity. It was first described by Harach et al. [1] in association with FAP as a distinctive tumor. A total of 44 cases have been documented so far (See Table 1). We describe three cases, in two of which the patients have FAP. Case 2 was lost to follow-up.
Two cases were women in their 30's and the third was a 14 year old. The lesions ranged from 1.5 to 2 cm and were associated with several satellite nodules. There was no lymph node metastasis, capsular or vascular invasion. Our cases are similar to that described by Cameselle-Teijeiro et al. [2] who first proposed the term and did a study on nine cases. All the cases were seen in women between ages of 16-30 years (mean: 21.3). The lesions were predominantly solitary and ranged from 1.5-5.6 cm. All except one showed vascular invasion. Two cases also showed lymph node metastasis. In that study, immunohistochemical stains were positive for thyroglobulin, epithelial membrane antigen, cytokeratin, vimentin, estrogen and progesterone receptors, bcl-2 and Rb proteins. Calcitonin and carcinoembryonic antigen were negative. Follow-up showed seven cases with no recurrences.
C-MV of PTC is commonly seen in young females usually less than 30 years of age. The lesions are encapsulated or well-circumscribed. While sporadic forms usually appear as an isolated tumors, the cases associated with FAP are often multifocal due to different somatic mutations added to the germline mutations [3]. They display the characteristic histologic pattern of cribriforming akin that seen in breast cancer with morules. Morules appear squamous with no keratinization or cellular bridges. There are also follicles showing papillary, trabecular and solid patterns.
There have been several cases showing germline mutations in the APC gene which have also been found in the colonic polyps. Hot spots on codons 1061, 1039 and 698 of the APC gene on exon 15 are frequently identified. It has been proposed that β-catenin immunohistochemistry is a feasible screening method to identify occult FAP in young patients with thyroid tumors [4]. Recently Xu B [5] suggested that accumulation of mutant β-catenin contributes to the development of C-MV of PTC. A handful of sporadic cases have also been documented [6]. Table 1 summarizes all the cases in the literature. Molecular studies were performed in all our cases and they did not show the hot spots of the APC gene.
C-MV carries a better prognosis than the other aggressive variants of PTC (tall cell, columnar, diffuse sclerosing and diffuse follicular) and poorly differentiated carcinoma. Tall cell variant lacks morular, cribriform and spindle cell areas. Columnar cell variant presents in older males. The encapsulated form of columnar cell variant is common in females and histologically shows greater overlap with C-MV but does not have the morules and cribriform pattern. Hyalinizing trabecular tumor shows a zellballen pattern in a hyalinized amyloid-like background. Poorly differentiated (insular) carcinoma shows areas mimicking cribriform structures but lacks morules and is associated with a higher proliferation index and necrosis.
This morphologic variant should be borne in mind by pathologists because of its characteristic pattern. The clinician should be alerted to exclude FAP along with appropriate family screening. In 25-30% of cases, this might provide the first indicator of an underlying FAP syndrome.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
1. Harach HR, Williams GT, Williams E.D. Familial adenomatous polyposis associated thyroid carcinoma: a distinct type of follicular cell neoplasm. Histopathology. 1994 ;6 :549 -561
2. Cameselle-Teijeiro J, Chan J.K. Cribriform-morular variant of papillary carcinoma: a distinctive variant representing the sporadic counterpart of familial adenomatous polyposis-associated thyroid carcinoma? Mod Pathol. 1999 ;4 :400 -411
3. Miyaki M. et al. Molecular evidence for multicentric development of thyroid carcinomas in patients with familial adenomatous polyposis. Am J Pathol. 2000 ;6 :1825 -1827
4. Kurihara K. Nuclear localization of immunoreactive b-catenin is specific to familial adenomatous polyposis in papillary thyroid carcinoma. Jpn J Cancer Res. 2000 ;91 :1100 -1102
5. Xu B. et al. Cribriform-morular variant of papillary thyroid carcinoma: a pathological and molecular genetic study with evidence of frequent somatic mutations in exon 3 of the beta-catenin gene. J Pathol. 2003 ;1 :58 -67
6. Cameselle-Teijeiro J. et al. Somatic but not germline mutation of the APC gene in a case of cribriform-morular variant of papillary thyroid carcinoma. Am J Clin Pathol. 2001 ;115 :486 -493
7. Yamashita T. et al. Peculiar nuclear clearing composed of microfilaments in papillary carcinoma of the thyroid. Cancer. 1992 ;70 (12) :2923 -2928
8. Mizukami Y. et al. Encapsulated follicular thyroid carcinoma exhibiting glandular and spindle cell components: A case report. Pathol Res Pract. 1996 ;192 (1) :72 -74
9. Hizawa K. et al. Association between thyroid cancer of cribriform variant and familial adenomatous polyposis. J Clin Pathol. 1996 ;49 (7) :611 -613
10. Perrier N.D. et al. Thyroid cancer in patients with familial adenomatous polyposis. World J Surg. 1998 ;22 (7) :738 -743
11. Soravia C. et al. Familial adenomatous polyposis-associated thyroid cancer: a clinical, pathological, and molecular genetics study. Am J Pathol. 1999 ;154 (1) :127 -135
12. Fenton P.A. et al. Cibriform variant papillary thyroid cancer: a characteristic of familial adenomatous polyposis. Thyroid. 2001 ;11 (2) :193 -97
13. Cetta F. et al. Thyroid carcinoma associated with familial adenomatous polyposis. Histopathology. 1997 ;31 (3) :231 -236
Tables and Figures
| Cases | Sex/Age (yr) | FAP | Presentation | Main Features | APC Mutation in thyroid | Treatment | Outcome |
| Harach et al. [1] | F/19 | Yes | Right neck mass | 16 separate nodules | ND | Total thyroidectomy | A&W at 25 yrs |
| F/34 | Yes | 2 cm | ND | ||||
| F/34 | Yes | 1 focus | ND | ||||
| F/23 | Yes | 3 cm | ND | ||||
| Cameselle-Teijeiro and Chan [2] | F/30 | No | Right neck nodule at 6 months | 22 mm | Absent | Hemithroidectomy (R), Subtotal lobectomy (L), Radioactive iodine | A&W at 5 yr |
| F/16 | No | Left neck nodule for 1 year | 15 mm | ND | Total thyroidectomy, Radioactive iodine | A&W at 14 yr | |
| F/20 | No | Left thyroid nodule | 12 mm (R), 23 mm (L) | ND | Total thyroidectomy | A&W at 4 yr | |
| F/19 | No | Right neck mass | 19mm | ND | Total thyroidectomy | Recent case | |
| Yamashita et al.[7] | F/21 | No | Anterior neck mass | 40 mm | ND | Surgical resection | A&W at 1-2 yr |
| F/23 | No | Anterior neck mass | 50 mm, LN met + | ND | Surgical resection | A&W at 1-2 yr | |
| F/20 | No | Anterior neck mass | 56 mm | ND | Surgical resection | A&W at 1-2 yr | |
| F/21 | No | Anterior neck mass | 26 mm | ND | Surgical resection | A&W at 1-2 yr | |
| Mizukami et al.[8] | F/16 | No | Left neck nodule | 20 mm | Subtotal thyroidectomy and radical neck dissection | A&W at 5 yr | |
| Hizawa et al.[9] | F/20 | Yes | Right neck mass | 18 mm | Exon 15 at codon 1061 | Hemithroidectomy (R), and LN dissection | A&W at 2 yr |
| Perrier et al.[10] 11/12 cases | F/15-61 yrs | Yes | Bilateral : 5 | 18 mm (0.2-5 cm) multicentric :8 2 cases with LN met's | All ND | 5 Total thyroidectomy 5 near total thyroidectony 2 lobectomy 2 PO Iodine 10 T4 suppression | FU:&mos-30yr, 2 recurred 1 death |
| Soravia et al.[11] | F/38 | Yes | Bilateral mass | Multifocal (3-35 mm) capsular and vascular + | Exon 15 at codon 698 | Total thyroidectomy | Recurred and died |
| F/24 | Yes | Bilateral mass | 20-35 mm, capsular invasion | Exon 15 at codon 698 | Total thyroidectomy | Recurred | |
| F/51 | Yes | Bilateral mass | 6-23 mm | Exon 9 at codon 312 | Total thyroidectomy | NP | |
| Fenton et al.[12] | F/20 | Yes | Diagnosis made retrospectively | Diagnosis made retrospectively | Exon 15 at codon 1061 | Total thyroidectomy | A&W at 14mos |
| Cameselle-Teijeiro et al. [6] | F/27 | Yes | Right neck mass x 14 mos | 21 mm with capsular and angioinvasion | Exon 15 at codon 1309 somatic mutation | Total thyroidectomy | A&W at 14mos |
| Cetta et al.[13] | F/22 | Yes | NP | Encapsulated | Exon 15 at codon 1061 | NP | NP |
| F/20 | Yes | NP | 8 mm | Exon 15 at codon 1309 | NP | NP | |
| F/36 | Yes | NP | 11 mm | Exon 15 at codon 1309 | NP | NP | |
| Present Cases | F/32 | Yes | Anterior neck | Multifocal | In process | Total thyroidectomy | Recent case |
Author biography
Shylashree Chikkamuniyappa, MD, is a final year Anatomic/Clinical pathology resident at Univ TX Hlth Sci Ctr at San Antonio. She is serving as the Chief resident in the department and serving on various institutional and national committees. She is currently working on several research projects with faculty and presented at national meetings. She will pursue her fellowship in hematopathology for the next 2 years.
Jaishree Jagirdar, MD, is the Professor and Director of Anatomic Pathology at the Uni Tx Hlth Sci Ctr at San Antonio. After completing her residency in AP/CP at Mt. Sinai Medical Center in New York, she stayed on as faculty for 10 years. Following that she worked at New York University for another 11 years. Her area of expertise is in Pulmonary Pathology, both diagnostic and research aspects. She is the Hlth Sci Ctr Pulmonary Pathology Consultant and has published primarily on lung diseases for the last 20 years.
![]() Corresponding address:
Corresponding address:
Shylashree Chikkamuniyappa, MD, Department of Pathology, UTHSCSA Mail Code 7750, 7703 Floyd Curl Drive, San Antonio, Texas 78229-3900. Tel: (210) 567-6731 Fax: (210) 692-1480 Email: chikkamuniyaedu

 Global reach, higher impact
Global reach, higher impact