3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2024; 21(4):714-724. doi:10.7150/ijms.91894 This issue Cite
Research Paper
The DNA-dependent protein kinase catalytic subunit promotes sepsis-induced cardiac dysfunction through disrupting INF-2-dependent mitochondrial dynamics
1. Shenshan Medical Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Shanwei, Guangdong, China.
2. Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
3. Senior Department of Cardiology, The Sixth Medical Center of People's Liberation Army General Hospital, Beijing, China.
#The first two authors contributed equally to this article.
Abstract
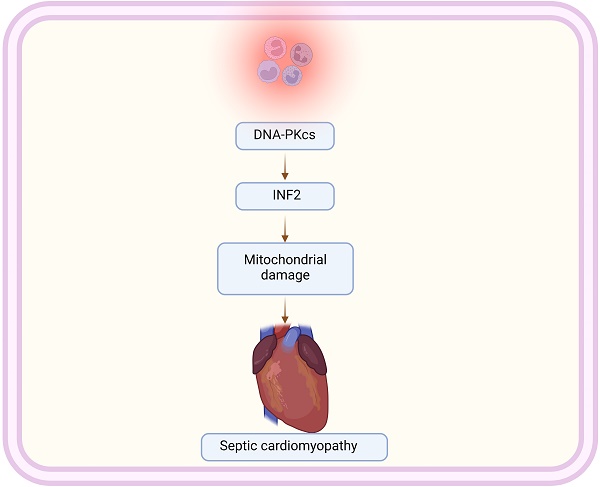
Sepsis-induced cardiomyopathy (SIC) represents a severe complication of systemic infection, characterized by significant cardiac dysfunction. This study examines the role of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) and Inverted Formin 2 (INF2) in the pathogenesis of SIC, focusing on their impact on mitochondrial homeostasis and dynamics. Our research demonstrates that silencing DNA-PKcs alleviates lipopolysaccharide (LPS)-induced cardiomyocyte death and dysfunction. Using HL-1 cardiomyocytes treated with LPS, we observed that DNA-PKcs knockdown notably reverses LPS-induced cytotoxicity, indicating a protective role against cellular damage. This effect is further substantiated by the reduction in caspase-3 and caspase-9 activation, key markers of apoptosis, upon DNA-PKcs knockdown. Besides, our data further reveal that DNA-PKcs knockdown attenuates LPS-induced mitochondrial dysfunction, evidenced by improved ATP production, enhanced activities of mitochondrial respiratory complexes, and preserved mitochondrial membrane potential. Moreover, DNA-PKcs deletion counteracts LPS-induced shifts towards mitochondrial fission, indicating its regulatory influence on mitochondrial dynamics. Conclusively, our research elucidates the intricate interplay between DNA-PKcs and INF2 in the modulation of mitochondrial function and dynamics during sepsis-induced cardiomyopathy. These findings offer new insights into the molecular mechanisms underpinning SIC and suggest potential therapeutic targets for mitigating mitochondrial dysfunction in this critical condition.
Keywords: DNA-PKcs, INF2, LPS, mitochondrial homeostasis

 Global reach, higher impact
Global reach, higher impact