ISSN: 1449-1907International Journal of Medical Sciences
Int J Med Sci 2024; 21(4):601-611. doi:10.7150/ijms.91506 This issue Cite
Research Paper
Enhanced Diagnostic Efficiency of Endometrial Carcinogenesis and Progression in Women with Abnormal Uterine Bleeding through Peripheral Blood Cytokine Testing: A Multicenter Retrospective Cohort Study
1. Laboratory of Gynecologic Oncology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou 350001, Fujian, China.
2. Fujian Key Laboratory of Women and Children's Critical Diseases Research, Fujian Maternity and Child Health Hospital, Fuzhou 350001, Fujian, China.
3. Fujian Clinical Research Center for Gynecological Oncology, Fujian Maternity and Child Health Hospital, Fuzhou 350001, Fujian, China.
4. Department of Gynecology, Fujian Maternity and Child Health Hospital, College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou 350001, Fujian, China.
5. Zhangzhou Affiliated Hospital of Fujian Medical University, Zhangzhou 363000, Fujian, China.
6. Department of Obstetrics and Gynecology, The First Affiliated Hospital, Fujian Medical University, Fuzhou 350005, Fujian, China.
7. Department of Obstetrics and Gynecology, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou 350212, Fujian, China.
8. The Second Hospital of Nanping City, Nanping 354200, Fujian, China.
* These authors contributed equally to this work.
Abstract
Objective: This study aimed to evaluate the role of plasma cytokine detection in endometrial cancer screening and tumor progression assessment in patients with abnormal uterine bleeding.
Methods: In this multicenter retrospective cohort study of 287 patients with abnormal uterine bleeding, comprehensive clinical information and laboratory assessments, including cytokines, routine blood tests, and tumor markers, were performed. Associations between the clinical indicators and endometrial carcinogenesis/progression were evaluated. The independent risk factors for endometrial cancer and endometrial cancer with deep myometrial invasion were analyzed using multivariate binary logistic regression. Additionally, a diagnostic model was used to evaluate the predictive efficacy of these identified risk factors.
Results: In patients with abnormal uterine bleeding, low IL-4 and high IL-8 levels were independent risk factors for endometrial cancer (p < 0.05). Combining IL-4, IL-8, CA125, and menopausal status improved the accuracy of assessing endometrial cancer risk. The area under curve of the model is 0.816. High IL-6 and IL-8 levels were independent risk factors for deep myometrial invasion in patients with endometrial cancer (p < 0.05). Similarly, combining IL-6, IL-8, and Monocyte counts enhanced the accuracy of assessing endometrial cancer risk with deep myometrial invasion. The area under curve of the model is 0.753.
Conclusions: Cytokines such as IL-4, IL-8, and IL-6 can serve as markers for monitoring endometrial cancer and its progression in women with abnormal uterine bleeding.
Keywords: endometrial cancer, cytokine, abnormal uterine bleeding, biomarker, inflammation, predictive model.
Introduction
Endometrial cancer (EC) is the most prevalent gynecological malignancy globally [1]. In 2020, China witnessed 81,964 newly reported EC cases, resulting in 16,607 fatalities [2, 3]. Globally, the annual incidence of EC has been increasing year by year [4,5]. Over 90% of EC patients manifest varying forms of abnormal vaginal bleeding as a primary symptom, encompassing postmenopausal vaginal bleeding and menstrual irregularities [6]. Abnormal uterine bleeding (AUB), a prevalent symptom in obstetrics and gynecology [7]. Among women aged 45 and older, those with AUB have a certain risk of EC [8]. Distinguishing AUB from EC is of paramount importance for facilitating appropriate patient management.
Although procedures such as hysteroscopy, curettage, and endometrial biopsy exhibit high diagnostic accuracy, their invasive nature and associated discomfort, including intense pain, pose challenges [9, 10]. The quest for a novel, non-invasive, and cost-effective diagnostic tool is imperative to mitigate unnecessary curettage. Peripheral blood samples, characterized by accessibility and relative noninvasiveness, have emerged as promising repositories for potential biomarkers. Although existing serum tumor markers such as HE4 and CA125 play a role in EC diagnosis, their sensitivity and specificity remain unclear [11, 12, 13]. Cytokines, pivotal modulators of immune responses, offer heightened sensitivity compared to other immune factors. Previous studies have shown that increased serum levels of pro-inflammatory cytokines are associated with tumor metastasis and progression [14, 15].
However, screening methods employing immune blood biomarkers for prognosticating EC risk in patients with AUB are lacking. In response to this gap, our study propounds a pioneering approach that integrates blood cytokine testing to assess the propensity of EC incidence and progression in perimenopausal patients with AUB. This initiative aims to increase diagnostic precision and alleviate the clinical discomfort associated with diagnosis and treatment. Further, this study provides innovative avenues for advancing anti-inflammatory strategies and immunotherapeutic approaches in tumor management.
Materials and Methods
Ethics statement
Ethical approval was obtained from the Ethics Committee of Fujian Provincial Maternity and Children's Hospital (2022KYLLR03022). The requirement for informed consent was waived due to the retrospective nature of the study. The study protocol conformed with the principles of the Declaration of Helsinki.
Study population
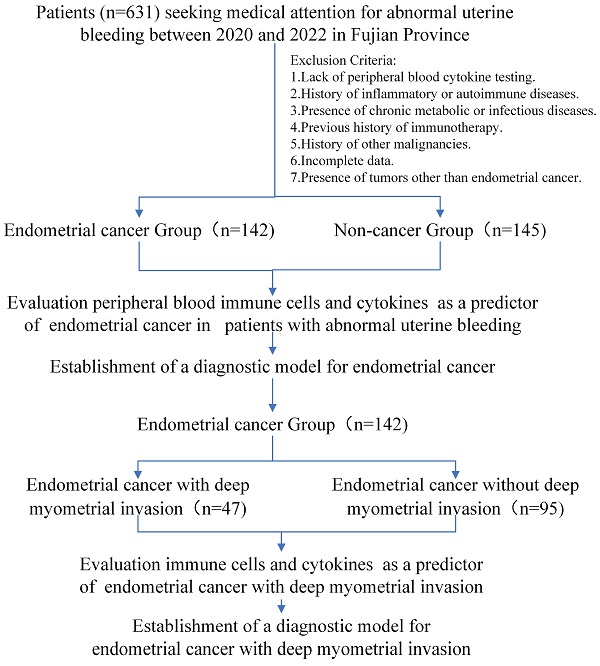
This retrospective cohort study was conducted at multicenter and included 287 patients aged 45 and older who presented to hospitals with AUB and underwent peripheral blood immune indicator testing from 2020 to 2022 in Fujian Province. Exclusion criteria included a history of inflammatory disease, autoimmune disease, chronic metabolic disease, chronic infectious disease, prior history of immunotherapy and other malignancies, and loss to follow-up. All patients underwent endometrial biopsy or curettage. Among them, 142 were diagnosed with EC. Within this group, 47 were associated with deep myometrial invasion (DMI), whereas 95 were not. The remaining 145 patients were diagnosed with benign uterine diseases.
Clinical laboratory data
All patients underwent comprehensive clinical laboratory examinations, including assessments of cytokines, routine blood tests, and tumor markers. Data on risk factors for EC such as age, body mass index (BMI), and menopausal status were systematically collected. Additionally, histopathological findings were included in the study.
Statistical analysis
Data analysis was performed using SPSS version 20.0, and R version 4.2.1. Continuous data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Student's t-test or Mann-Whitney U test was used to analyze the differences between groups. The chi-squared test or Fisher's exact test was used for categorical data, which were presented as counts with frequencies. Receiver Operating Characteristic (ROC) curve analysis was performed, and the ROC area under the curve (AUCs) was used to evaluate the diagnostic accuracy of EC. Logistic regression analysis was employed to identify independent risk factors and to establish and evaluate a diagnostic model. The significance level was set at p < 0.05.
Results
General characteristics between EC and Non-EC groups
A flowchart of the study is depicted in Figure 1. The outcomes unveiled a distinct pattern among perimenopausal individuals seeking medical care for AUB. Those diagnosed with EC tended to be of advanced age, predominantly in the postmenopausal phase (Table 1). Notably, the blood tumor marker CA125 was elevated in patients with EC (p < 0.05). This observation suggests a role for CA125 levels in evaluating the risk of EC in individuals presenting with AUB.
Baseline characteristics of EC Group and Non-cancer Group in study participants.
| Characteristics | EC Group | Non-cancer Group | P value | |
|---|---|---|---|---|
| N | 142 | 145 | ||
| Age, median (IQR) | 55 (51, 61) | 52 (48, 58) | 0.006 | |
| BMI, median (IQR) | 24.321 (22.022, 27.441) | 23.712 (21.644, 26.839) | 0.379 | |
| Menopause status, n (%) | YES | 92 (32.1%) | 64 (22.3%) | <0.001 |
| NO | 50 (17.4%) | 81 (28.2%) | ||
| History of hypertension, n (%) | YES | 46 (16%) | 39 (13.6%) | 0.289 |
| NO | 96 (33.4%) | 106 (36.9%) | ||
| History of diabetes, n (%) | YES | 26 (9.1%) | 25 (8.7%) | 0.791 |
| NO | 116 (40.4%) | 120 (41.8%) | ||
| CA125, median (IQR) | 20 (11.975, 41.425) | 17.2 (11.55, 27.25) | 0.021 |
Note: p < 0.05 was considered to be statistically significant. Abbreviations: BMI, body mass index; CA125, cancer antigen 125.
Study cohort flowchart.

Independent risk factors for endometrial carcinogenesis in AUB patients
Our investigation included the assimilation of routine blood test data and cytokine profiles from a patient cohort. The assessment of neutrophils, lymphocytes, and monocytes levels revealed no statistically significant differences between the EC and non-cancerous groups. This observation implies a limited role for immune cells in routine blood assessments of tumors. Conversely, in the EC group, the pro-inflammatory marker IL-8 exhibited a notable increase (p < 0.05), whereas the anti-inflammatory cytokine IL-4 displayed a distinct decrease (p<0.05), and these disparities were statistically significant (Figure 2). The statistically significant indicators were subsequently evaluated by dichotomizing continuous data using ROC curve analysis and the Youden Index (Figure 3A-D). Cutoff values were established for significant EC group indicators, including age (cutoff = 53.5), IL-4 (cutoff =1.95), IL-8 (cutoff =7.405), and CA125 (cutoff =33.8). Comprehensive univariate and multivariate logistic regression analyses were then performed to determine baseline characteristics and clinicopathological features. The resultant outcomes revealed that menopausal status and IL-4, IL-8, and CA125 levels were independent risk factors for the EC-group (all p < 0.05, Figure 3E). These independent risk factors were harnessed to construct a nomogram tailored for the EC group, thus offering a quantitative prediction avenue (Figure 4A). Calibration plots corroborated a commendable alignment between actual observations and predicted probabilities, substantiated by a Hosmer-Lemeshow P-value of 0.8585 (Figure 4B). Concurrently, the ROC curve analysis indicated that, when compared to individual indices, the amalgamation of menopausal status, IL-4, IL-8, and CA125 indices significantly augmented the diagnostic efficacy for EC patients (AUC=0.816, p < 0.001, Figure 4C).
Characteristics of peripheral blood immune cells and cytokines among study participants, comparing the endometrial cancer and Non-cancer group. A: The peripheral blood immune cells between endometrial cancer and Non-cancer group; B: The peripheral blood cytokines between endometrial cancer and Non-cancer group. Note: *, p < 0.05; ***, p < 0.001; p<0.05 indicates significant differences. Abbreviations: WBC, white blood cell; NE, neutrophils; Mo, monocyte; Lym, lymphocyte; IL, Interleukin.

High risk factors and predictive models for endometrial cancer in patients with abnormal uterine bleeding during perimenopause. A-D: Receiver operating characteristic (ROC) curves for Age, IL-4, IL-8, and CA125. The cut-off values for these indicators were also determined (Age: Cutoff=53.5, IL-4: Cutoff=1.95, IL-8: Cutoff=7.405, and CA125: Cutoff=33.8); E: Logistic regression analyses for EC.

Predictive models for endometrial cancer in patients with abnormal uterine bleeding during perimenopause. A: Nomogram for predicting endometrial cancer; B: Calibration plots for the nomogram with Hosmer-Lemeshow P=0.8585; C: ROC curve for the nomogram. (AUC=0.816, p < 0.001).

Baseline characteristics of endometrial cancer group participants stratified by deep muscle invasion and non-deep muscle invasion.
| Characteristics | DMI | NDMI | P value | |
|---|---|---|---|---|
| N | 47 | 95 | ||
| Age, mean ± sd | 57.213 ± 9.5734 | 55.211 ± 7.8482 | 0.186 | |
| BMI, median (IQR) | 24.524 (22.49, 27.319) | 24.051 (21.967, 27.584) | 0.862 | |
| Menopause status, n (%) | Yes | 30 (21.3%) | 61 (43.3%) | 0.901 |
| No | 17 (12.1%) | 33 (23.4%) | ||
| History of hypertension, n (%) | Yes | 16 (11.3%) | 30 (21.3%) | 0.799 |
| NO | 31 (22%) | 64 (45.4%) | ||
| History of diabetes, n (%) | Yes | 7 (5%) | 19 (13.5%) | 0.443 |
| NO | 40 (28.4%) | 75 (53.2%) | ||
| FIGO stage, n (%) | Ⅰ~Ⅱ | 36 (25.4%) | 95 (66.9%) | < 0.001 |
| Ⅲ~Ⅳ | 11 (7.7%) | 0 (0%) | ||
| Grade, n (%) | G1 G2 G3 | 22 (15.5%) 19 (13.4%) 6 (4.2%) | 76 (53.5%) 13 (9.2%) 6 (4.2%) | < 0.001 |
| Deep muscle invasion, n (%) | Yes | 47 (33.1%) | 0 (0%) | < 0.001 |
| No | 0 (0%) | 95 (66.9%) | ||
| Lymph node metastasis, n (%) | No | 34 (23.9%) | 95 (66.9%) | < 0.001 |
| Yes | 13 (9.2%) | (0%) | ||
| ER, n (%) | - ~+ | 33 (23.2%) | 54 (38%) | 0.124 |
| ++~+++ | 14 (9.9%) | 41 (28.9%) | ||
| PR, n (%) | - ~+ | 35 (24.6%) | 56 (39.4%) | 0.070 |
| ++~+++ | 12 (8.5%) | 39 (27.5%) | ||
| Ki67, median (IQR) | 0.4 (0.3, 0.6) | 0.4 (0.3, 0.4) | 0.106 | |
| CA125, median (IQR) | 21.6 (15.24, 42.9) | 18.27 (11.1, 40.325) | 0.232 |
Note: p<0.05 was considered to be statistically significant. Abbreviations: DMI, deep muscle invasion; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; ER, estrogen receptor; PR, progesterone receptor; CA125, cancer antigen 125.
General characteristics between EC with and without deep myometrial invasion
The primary mode of metastasis in EC involves direct spread, with cancer cells infiltrating the myometrium, leading to further expansion, invasion, and subsequent metastasis. Consequently, the categorization of patients with EC into those with and without deep myometrial invasion serves as a means of investigating risk factors for tumor progression. Basic data pertaining to EC cases with and without deep myometrial invasion are detailed in Table 2. Notably, a statistically significant difference were observed between the two groups in terms of Federation International of Gynecology and Obstetrics (FIGO) stage, grading, and lymph node metastasis. These indicators directly correlated with deep myometrial invasion and tumor progression, thus underscoring their limited research value. Conversely, CA125 levels were not significantly different between the two groups. This observation suggests a restricted role of CA125 in determining the trajectory of tumor progression.
Independent risk factors for EC with deep myometrial invasion and without patients with AUB
Compared to cases of EC devoid of deep myometrial invasion, both monocyte count and percentage were considerably elevated in EC cases characterized by deep myometrial invasion. Additionally, within the subset of EC cases marked by deep myometrial invasion, elevated levels of the pro-inflammatory markers IL-6 and IL-8 were apparent. All the observed differences were statistically significant (Figure 5). The highlighted significant parameters were subsequently subjected to further analysis. Continuous data were dichotomized for subsequent analyses using ROC curve analysis and the Youden Index (Figure 6A-D). Consequently, distinct cutoff values were established for monocyte count (MO) (cutoff =0.445), MO % (cutoff =7.05), IL-6 (cutoff =4.15), and IL-8 (cutoff =10.675).
Characteristics of peripheral blood immune cells and cytokines between endometrial cancer with deep myometrial invasion and without deep myometrial invasion in study participants. A: The peripheral blood immune cells between endometrial cancer with and without deep myometrial invasion; B: The peripheral blood cytokines between endometrial cancer with and without deep myometrial invasion. Note: *, p < 0.05; ***, p < 0.05; p<0.05 indicates significant differences. Abbreviations: WBC, white blood cell; NE, neutrophils; Mo, monocyte; Lym, lymphocyte; IL, interleukin.

High risk factors for endometrial cancer with deep myometrial invasion in patients with abnormal uterine bleeding. A-D: Receiver operating characteristic (ROC) curves for IL-6, IL-8, Mo, and Mo. The cut-off values for these indicators were also determined (MO: Cutoff=0.445), MO%: Cutoff=7. 05, IL-6: Cutoff=4.15, and IL-8: Cutoff=10.675). E: Logistic regression analyses for endometrial cancer with deep myometrial invasion.

Univariate and multivariate logistic regression analyses were performed for all baseline and clinicopathological features. The outcomes underscored the independence of MO, IL-6, and IL-8 as risk factors for EC with deep myometrial invasion (all p < 0.05; Figure 6E). These significant indicators were used to construct a nomogram tailored to predict EC cases featuring deep myometrial invasion, offering a quantitative prediction avenue (Figure 7A). Notably, calibration plots affirmatively demonstrated robust consistency between actual observations and predicted probabilities, as evidenced by a Hosmer-Lemeshow P-value of 0.8102 (Figure 7B). Concurrently, ROC curve analysis highlighted the potency of combining MO, IL-6, and IL-8 over individual indices, significantly augmenting the diagnostic efficacy for EC cases with deep myometrial invasion (AUC=0.753, p < 0.001, Figure 7C).
Discussion
AUB is a prevalent symptom that demands attention in women's health and is frequently associated with the potential risk of EC. Regrettably, existing screening approaches are either invasive or lack the requisite specificity [16, 17]. Such diagnostic uncertainties inflict both physical and psychological distress on patients with AUB. A significant challenge for clinicians is distinguishing between benign AUB and EC through non-invasive methodologies.
In our study, the analysis of cytokine test data, particularly IL-8, IL-4, and IL-6 levels, proved noteworthy in the diagnosis of EC among individuals with AUB. In patients with AUB, low IL-4 and high IL-8 levels were identified as independent risk factors for EC. Combining IL-4, IL-8, CA125, and menopausal status enhanced the accuracy of EC risk assessment. Furthermore, high IL-6 and IL-8 levels were independent risk factors for deep myometrial invasion in patients with EC. The combination of IL-6, IL-8, and monocyte counts could provide a more accurate assessment of the risk of EC with deep myometrial invasion.
The role of inflammation in tumorigenesis and tumor development has received extensive attention, as it influences tumor initiation, growth, progression, and metastasis [18]. IL-6 and IL-8 are known pro-inflammatory cytokines, whereas IL-4 is an anti-inflammatory cytokine [19]. Our research indicates that the pro-inflammatory factor IL-8 was elevated in patients with EC. Studies have demonstrated increased levels of IL-8 in various tumor tissues and patient serum, including breast cancer, nasopharyngeal carcinoma, colorectal cancer, and gastric cancer [20]. Numerous previous studies have demonstrated increased levels of IL-8 in patients with EC when compared in the normal population [21, 22, 23]. Kotowicz et al. suggested that high levels of IL-8 in the serum can serve as an indicator for distinguishing patients with EC from the normal population, with a diagnostic sensitivity of 0.818 [21].
Predictive models for EC with deep myometrial invasion in EC patients. A: Nomogram for predicting endometrial cancer with deep myometrial invasion. B: Calibration plots for the nomogram, Hosmer-Lemeshow P=0.8102. C: ROC curve for the nomogram, (AUC=0.753, p < 0.001).

The high expression of IL-8 is not only related to the occurrence of EC but also to its progression and prognosis. Previous studies have shown a statistically significant correlation between EC myometrial invasion and IL-8 levels [23, 24]. IL-8 appears to function as an angiogenic switch during myometrial invasion in stage I uterine ECs [23, 24]. Thus, it may serve as a valuable marker for stratifying patients with EC based on lymphovascular invasion and EC grade [23]. Similarly, our research indicate that IL-8 was an independent risk factor for deep myometrial invasion in patients with EC. Kotowicz et al. [21] demonstrated the clinical utility of IL-8 measurements as potential prognostic factors in type 1 EC. In that study, elevated pretreatment IL-8 serum levels were independently associated with shorter disease-free and overall survival. Furthermore, a direct link between high serum IL-8 concentrations and disease progression, such as tumor size, stage, and prognosis, has been reported in patients with breast, colon, ovarian, prostate, and melanoma [25, 26]. These associations may be attributed to IL-8's role in promoting angiogenesis, cancer stem cell survival signaling, and immunosuppression [27].
In our study, IL-6, another pro-inflammatory cytokine, exhibited increased expression in the EC deep myometrial invasion group. Serum IL-6 levels were an independent risk factor for poor prognosis. This result is consistent with other findings that IL-6 might significantly predict cancer progression in colorectal, prostate, and breast cancer [28]. High serum IL-6 levels might promote the progression of EC, possibly related to IL-6-induced migration and invasion of EC cells via the Stat3 signaling pathway [29]. Additionally, previous studies have shown that IL-6 promotes EC growth through an autocrine feedback loop involving ERK-NF-κB signaling pathway [30]. In addition to promoting the EC cell growth, IL-6 is upregulated in EGFR-mutant non-small cell lung cancer, where it suppresses T- and NK-cell functions. IL-6 blockade enhances antitumor immunity and the efficacy of anti-PD-1 therapy [31].
IL-4, a potent regulator of antitumor immune responses, possesses both tumor-promoting and tumor-inhibiting properties due to its immunosuppressive and anti-angiogenic functions [32, 33]. In our study, we observed a decrease in IL-4 levels in patients with EC. Consistent with our findings, IL-4 is also identified to exert an inhibitory influence on the proliferation of renal, colon, and breast cancer cells. This influence further elicits regression in specific tumor xenografts within murine cancer models [34]. Similarly, certain studies have shown that IL-4 has anti-inflammatory effects and may decrease the risk of esophageal squamous cell carcinoma by inhibiting inflammation [35, 36]. In contrast, some studies have shown that higher serum IL-4 levels correlate with advanced cancers [37, 38]. However, studies on the role of IL-4 in EC are limited. Additionally, polymorphisms of the IL-4 gene also determine its complex role in tumors, necessitating more detailed research.
The reduction of the anti-inflammatory cytokine IL-4 and the elevation of the pro-inflammatory factors L-6 and IL-8 in EC contributed to understand the intricate relationship between inflammation and cancer. Therefore, these changes could serve as monitoring indices for endometrial carcinogenesis and progression in women with AUB. Numerous studies have shown the pivotal role of other cytokines in the diagnosis and treatment of EC. Anti-inflammatory cytokines, including IL-10, TGF-β, IL-1Ra, and IDO, facilitate the evasion of immune attacks by the tumor. They establish an immunomodulatory environment, effectively suppressing the immune system. Pro-inflammatory cytokines such as IL-6, IL-8, IL-31, and IL-33 promote tumor growth and resistance to apoptosis [39]. Dinkic et al. demonstrated the potential involvement of cytokine IL-6 along with chemokines CCL5 and CCL2 in contributing to chemoresistance in EC [40]. Thus, cytokines have emerged as important targets for the diagnosis and treatment of EC.
Monocytes play an important role in various inflammatory responses in the body, including acute and chronic inflammation, tumors, and immune inflammation. Monocytes participate in the transmission of inflammatory signals and the generation and secretion of inflammatory molecules, thereby affecting the development of inflammatory reactions [41]. In our study, monocyte counts were considerably elevated in EC cases characterized by deep myometrial invasion. Matsuo et al. documented a significant correlation linking elevated monocyte counts to heightened risks of deep myometrial tumor invasion, pelvic lymph node metastasis, advanced-stage disease, and reduced survival rates in patients diagnosed with EC [42]. Analogous correlations between elevated circulating monocyte counts and diminished survival have been noted in additional gynecological malignancies, encompassing ovarian and cervical cancers [43]. Moreover, patients diagnosed with EC have displayed altered functionality in circulating monocytes, notably exhibiting heightened expression of the vascular endothelial growth factor (VEGF) receptor 1 on their cell surface. VEGF, produced by the tumor, exerts paracrine effects on macrophages, shaping them into tumor-associated macrophages (TAMs) in the local tumor microenvironment. Additionally, VEGF potentially have distant effects that modulate global monocyte functions in EC [44].
Numerous cancers either originate from chronic inflammation or prompt an inflammatory reaction (termed tumor-elicited inflammation), thereby establishing inflammation as a facilitating hallmark of cancer [18, 45]. Inflammation contributes to carcinogenesis by directly inducing mutagenesis or stimulating cytokine responses [18]. Further, cytokines recruit monocytes to become TAMs, with monocytic macrophages being the main immune cells producing cytokines. This, in turn, amplifies the role of inflammation.
Cumulatively, these findings accentuate the inflammatory backdrop associated with patients susceptible to EC, where inflammation potentially serving as a catalyst for cancer development. Cytokines play a vital role in mediating inflammation and cancer development. Therefore, cytokines hold promise as potential biomarkers for the early screening and continuous monitoring of tumor progression in EC. The identification of peripheral blood cytokines yields valuable insights into the intricate interplay between inflammation, immunity, and tumorigenesis. This provides innovative avenues for advancing anti-inflammatory strategies and immunotherapy approaches in tumor management.
Acknowledging the limitations of this study is crucial. The inclusion of a limited sample size, compounded by the nascent adoption of cytokine testing in clinical practice, may have introduced bias. Consequently, cautious interpretation is necessary, underscoring the need for further exploration using expanded cohorts to validate our conclusions. Moreover, the retrospective design of our study mandates vigilance regarding potential biases and limitations in data collection and interpretation. A prospective approach could provide more robust evidence. In future studies, these limitations could be circumvented through the inclusion of a broader and more diverse patient cohort, accompanied by comprehensive data collection to facilitate external validation.
Conclusions
Cytokines such as IL-4, IL-8, and IL-6 can be used to monitor endometrial carcinogenesis and progression in women with AUB.
Abbreviations
AUB: abnormal uterine bleeding; EC: endometrial cancer; NK: natural killer; BMI: body mass index; SD: standard deviation; IQR: interquartile range; ROC: receiver operating characteristic; AUC: areas under the curve; BMI: body mass index; ORs: odds ratios; CIs: confidence intervals; WBC: white blood cell; NE: neutrophils; Mo: monocyte; Lym: lymphocyte; IL: interleukin; FIGO: international federation of gynecology and obstetrics; DMI: deep myometrial invasion; CA125: carbohydrate antigen 125; TCGA: The Cancer Genome Atlas; ER: estrogen receptor; PR: progesterone receptor; TAMs: tumor-associated macrophages.
Acknowledgements
The authors thank Fujian Maternity and Child Health Hospital, the First Affiliated Hospital of Fujian Medical University, Zhangzhou Affiliated Hospital of Fujian Medical University, Mindong Hospital of Ningde City, Longyan First Hospital, and Quanzhou First Hospital Affiliated to Fujian Medical University for clinical sample collection. We also thank Dr. Xia Zhang, Dr. Guifen Liu, Dr. Xueke Lin, Dr. Lili Zheng, Dr. Yao Tong, and Dr. Meimei Huang for helping with data collection. We thank technicians Yafang Kang, Yaxing Shi, Lixiang Huang, Sihui Wang, Guixing Shen, Nan Wang, Yuxuan Huang, Maotong Zhang and Dr. Ye Li for helping with clinical samples collection and clinical examination. At the same time, we thank Xiantaoxueshu (www.xiantao.love) and Keyankezhan (www.sci-inn.com) for help with figures and the statistical analyses. We would like to thank Editage (www.editage.cn) for English language editing. Above all, the authors are grateful to all the patients and investigators who contribute to this clinical research and the progress of medicine.
Funding
This study was funded by National Key Research and Development Program of China (Grant number: 2022YFC2704303); National Natural Science Foundation of China (Grant number: 82203739); Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number: 2020Y9160); Startup Fund for scientific research, Fujian Medical University (Grant number: 2021QH1172, 2022QH2045), Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2021Y9157).
Data availability statement
Data will be made available upon reasonable request. Please contact the corresponding authors.
Ethical approval
Ethical approval was obtained from the Ethics Committee of Fujian Maternity and Child Health Hospital (Fujian Women and Children's Hospital) (2022KYLLR03022).
Informed consent statement
Informed consent was waived due to the retrospective nature of the study. The study protocol conformed with the principles of the Declaration of Helsinki.
Author contributions
Pengming Sun, Feifeng Shi, and Dabin Liu provided the concept of the paper. Feifeng Shi, Xiaozhen Xu, Xiaoli Huang, Yuan Ren, Caiping Deng, and Jincheng Ma completed the data collection. Leyi Huang, Jincheng Ma and Dabin Liu conducted the data analysis. Pengming Sun, Feifeng Shi and Xiaodan Mao guided the experiment throughout the process. Dabin Liu, Jincheng Ma and Leyi Huang completed the writing of the first draft. Pengming Sun, Xiaodan Mao, Dabin Liu, Jincheng Ma and Leyi Huang revised and polished the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li A, Jiao Y, Yong KJ, Wang F, Gao C, Yan B. et al. SALL4 is a new target in endometrial cancer. Oncogene. 2015Jan2;34(1):63-72
2. Sun P, Shen Y, Wang T, He Y, Zhang Y, Tian W. et al. Distinct clinical and genetic mutation characteristics in sporadic and Lynch syndrome-associated endometrial cancer in a Chinese population. Cancer Epidemiology. 2021Aug;73:101934
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clinicians. 2022Jan;72(1):7-33
4. Yang X, Wang J. The Role of Metabolic Syndrome in Endometrial Cancer: A Review. Front Oncol. 2019Aug8;9:744
5. Mao X, Lei H, Yi T, Su P, Tang S, Tong Y. et al. Lipid reprogramming induced by the TFEB-ERRα axis enhanced membrane fluidity to promote EC progression. J Exp Clin Cancer Res. 2022Dec;41(1):28
6. Makker V, MacKay H, Ray-Coquard I, Levine DA, Westin SN, Aoki D. et al. Endometrial cancer. Nat Rev Dis Primers. 2021Dec9;7(1):88
7. Chodankar R, Critchley HOD. Biomarkers in abnormal uterine bleeding†. Biol Reprod. 2019Dec;101(6):1155-66
8. Pennant M, Mehta R, Moody P, Hackett G, Prentice A, Sharp S. et al. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG: Int J Obstet Gy. 2017Feb;124(3):404-11
9. Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019Sep;19(9):510-21
10. Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. Obstetrical & Gynecological Survey. 2018Dec;73(12):687-8
11. Moore RG, Brown AK, Miller MC, Badgwell D, Lu Z, Allard WJ. et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecologic Oncology. 2008Aug;110(2):196-201
12. Cymbaluk-Ploska A, Chudecka-Głaz A, Pius-Sadowska E, Sompolska-Rzechuła A, Machaliński B, Jagodzińska A. et al. Clinical importance of serum HE4 and MMP2 levels in endometrial cancer patients. OTT. 2017Jun;10:3169-75
13. Gao M, Gao Y. Value of preoperative neutrophil-lymphocyte ratio and human epididymis protein 4 in predicting lymph node metastasis in endometrial cancer patients. J Obstet Gynaecol Res. 2021Feb;47(2):515-20
14. Degos C, Heinemann M, Barrou J, Boucherit N, Lambaudie E, Savina A. et al. Endometrial Tumor Microenvironment Alters Human NK Cell Recruitment, and Resident NK Cell Phenotype and Function. Front Immunol. 2019Apr26;10:877
15. Lu W, He F, Lin Z, Liu S, Tang L, Huang Y. et al. Dysbiosis of the endometrial microbiota and its association with inflammatory cytokines in endometrial cancer. Int J Cancer. 2021Apr;148(7):1708-16
16. Sun Y, Liu X, Zhao F, Hu L. Value of detection of serum human epididymis secretory protein 4 and carbohydrate antigen 125 in diagnosis of early endometrial cancer of different pathological subtypes. OTT. 2015 May;1239
17. Wang Q, Wang Q, Zhao L, Han L, Sun C, Ma S. et al. Endometrial Cytology as a Method to Improve the Accuracy of Diagnosis of Endometrial Cancer: Case Report and Meta-Analysis. Front Oncol. 2019Apr24;9:256
18. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019Jul16;51(1):27-41
19. Mertowska P, Mertowski S, Smarz-Widelska I, Grywalska E. Biological Role, Mechanism of Action and the Importance of Interleukins in Kidney Diseases. Int J Mol Sci. 2022Jan7;23(2):647
20. Yuen KC, Liu LF, Gupta V, Madireddi S, Keerthivasan S, Li C. et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020May;26(5):693-8
21. Kotowicz B, Fuksiewicz M, Jonska-Gmyrek J, Berezowska A, Radziszewski J, Bidzinski M. et al. Clinical significance of pretreatment serum levels of VEGF and its receptors, IL- 8, and their prognostic value in type I and II endometrial cancer patients. PLoS One. 2017;12(10):e0184576
22. Ciortea R, Mihu D, Mihu CM. Association between visceral fat, IL-8 and endometrial cancer. Anticancer Res. 2014Jan;34(1):379-83
23. Roškar L, Pušić M, Roškar I, Kokol M, Pirš B, Smrkolj Š. et al. Models including preoperative plasma levels of angiogenic factors, leptin and IL-8 as potential biomarkers of endometrial cancer. Front Oncol. 2022;12:972131
24. Fujimoto J, Aoki I, Khatun S, Toyoki H, Tamaya T. Clinical implications of expression of interleukin-8 related to myometrial invasion with angiogenesis in uterine endometrial cancers. Ann Oncol. 2002Mar;13(3):430-4
25. Chen Y, Shi M, Yu GZ, Qin XR, Jin G, Chen P. et al. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol. 2012Mar14;18(10):1123-9
26. Todorović-Raković N, Milovanović J. Interleukin-8 in breast cancer progression. J Interferon Cytokine Res. 2013Oct;33(10):563-70
27. Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á. et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017Nov;60:24-31
28. Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M. et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014Feb;141(2):125-39
29. Che Q, Xiao X, Liu M, Lu Y, Dong X, Liu S. IL-6 promotes endometrial cancer cells invasion and migration through signal transducers and activators of transcription 3 signaling pathway. Pathol Res Pract. 2019Jun;215(6):152392
30. Che Q, Liu BY, Wang FY, He YY, Lu W, Liao Y. et al. Interleukin 6 promotes endometrial cancer growth through an autocrine feedback loop involving ERK-NF-κB signaling pathway. Biochem Biophys Res Commun. 2014Mar28;446(1):167-72
31. Patel SA, Nilsson MB, Yang Y, Le X, Tran HT, Elamin YY. et al. IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin Cancer Res. 2023Apr3;29(7):1292-304
32. Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Semin Cancer Biol. 2006Feb;16(1):80-8
33. Wang HW, Joyce JA. Alternative activation of tumor-associated macrophages by IL-4: priming for protumoral functions. Cell Cycle. 2010Dec15;9(24):4824-35
34. Suzuki A, Leland P, Joshi BH, Puri RK. Targeting of IL-4 and IL-13 receptors for cancer therapy. Cytokine. 2015Sep;75(1):79-88
35. Cabantous S, Ranque S, Poudiougou B. et al. Genotype combinations of two IL4 polymorphisms influencing IL-4 plasma levels are associated with different risks of severe malaria in the Malian population. Immunogenetics. 2015Jun;67:283-8
36. FitzGerald LM, Zhao S, Leonardson A, Geybels MS, Kolb S, Lin DW. et al. Germline variants in IL4, MGMT and AKT1 are associated with prostate cancer-specific mortality: An analysis of 12,082 prostate cancer cases. Prostate Cancer Prostatic Dis. 2018Jun;21(2):228-37
37. Perambakam SM, Srivastava R, Peace DJ. Distinct cytokine patterns exist in peripheral blood mononuclear cell cultures of patients with prostate cancer. Clin Immunol. 2005Oct;117(1):94-9
38. Wise GJ, Marella VK, Talluri G, Shirazian D. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000Sep;164(3 Pt 1):722-5
39. Azadehrah M, Vosoogh S, Azadehrah M. The roles and therapeutic applications of cytokines in endometrial cancer. J Reprod Immunol. 2022Aug;152:103652
40. Dinkic C, Kruse A, Zygmunt M, Schuetz F, Brucker J, Rom J. et al. Influence of Paclitaxel and Heparin on Vitality, Proliferation and Cytokine Production of Endometrial Cancer Cells. Geburtshilfe Frauenheilkd. 2017Oct;77(10):1104-10
41. Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011Oct;32(10):470-7
42. Matsuo K, Hom MS, Moeini A, Machida H, Takeshima N, Roman LD. et al. Significance of monocyte counts on tumor characteristics and survival outcome of women with endometrial cancer. Gynecol Oncol. 2015Aug;138(2):332-8
43. Lee YY, Choi CH, Sung CO, Do IG, Huh S, Song T. et al. Prognostic value of pre-treatment circulating monocyte count in patients with cervical cancer: comparison with SCC-Ag level. Gynecol Oncol. 2012Jan;124(1):92-7
44. Brooks N, Stojanovska L, Grant P, Apostolopoulos V, McDonald CF, Pouniotis DS. Characterization of blood monocyte phenotype in patients with endometrial cancer. Int J Gynecol Cancer. 2012Nov;22(9):1500-8
45. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022Jan;12(1):31-46
46. Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022Aug;23(8):1148-56
Author contact
![]() Corresponding authors: Feifeng Shi (ffshi63com) and Pengming Sun (fmsun1975edu.cn).
Corresponding authors: Feifeng Shi (ffshi63com) and Pengming Sun (fmsun1975edu.cn).
Received 2023-10-25
Accepted 2024-1-9
Published 2024-1-21
