ISSN: 1449-1907International Journal of Medical Sciences
Int J Med Sci 2021; 18(5):1179-1184. doi:10.7150/ijms.53655 This issue Cite
Research Paper
Timing of mTORI usage and outcomes in kidney transplant recipients
1. Division of Nephrology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
2. Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
3. Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
4. Department of Nursing, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
5. Faculty of Renal Care, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
6. Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
7. Department of Biochemistry, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
Abstract
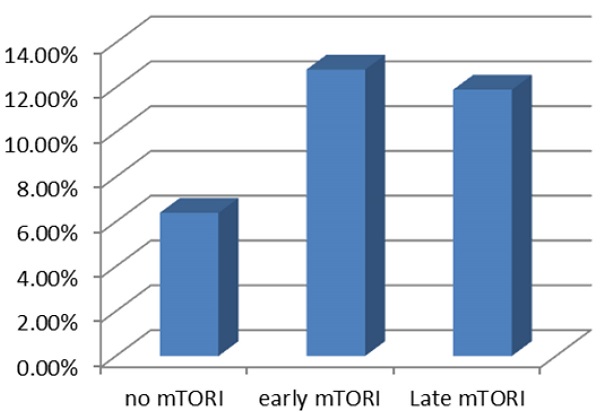
The introduction of mammalian target of rapamycin inhibitors (mTORi) as immunosuppressive agents has changed the landscape of calcineurin inhibitor-based immunosuppressive regimens. However, the timing of mTORi conversion and its associated outcomes in kidney transplantation have conflicting results. This study investigated the effect of early or late mTORi post-transplant initiation on major transplant outcomes, including post-transplant malignancy, in kidney transplant recipients in our center. We enrolled 201 kidney transplant recipients with surviving function grafts of >3 months between 1983 and 2016. Patients were divided into three groups: early mTORi (initiated within 6 months of kidney transplantation), late mTORi, (mTORi initiation >6 months after kidney transplantation) and no mTORi. The mean creatinine at conversion was 1.46 ± 0.48 mg/dL and 1.30 ± 0.53 mg/dL for the early and late mTORi groups, respectively. During the study period, 10.5% of mTORi users and 19.2% of mTORi nonusers developed malignancy, mainly urothelial carcinoma. After adjustment for confounding factors, mTORi users were found to have a lower incidence of post-transplant malignancy than did nonusers (adjusted OR: 0.28, P = 0.04). No significant difference was observed between early and late mTORi users. Our results verified the potential advantages of mTORi usage in reducing cancer incidence after kidney transplantation. However, no significant result was found related to the timing of mTORi introduction. Future studies should include a longer observation period with a larger cohort.
Keywords: kidney transplantation, immunosuppressant, mammalian target of rapamycin inhibitor (mTORI), outcomes, malignancy
